Abstract
Septic internal jugular vein-sigmoid sinus thrombosis (IJV-SST) associated with a malpositioned central venous catheter is a rare condition. It is potentially life-threatening and necessitates early diagnosis and rapid administration of appropriate medications. Unfortunately, it is difficult to diagnose due to vague clinical presentations. Several studies such as CT, MRI, and cerebral angiography should be performed and carefully examined to help make the diagnosis. We report a case of septic IJV-SST due to a malpositioned central venous catheter.
Septic internal jugular vein-sigmoid sinus thrombosis is a rare condition that complicates local and regional infectious inflammatory processes occurring in the head and neck. These include deep neck infections, Lemierre syndrome, and central venous catheterization (CVC) or cannulation17). These clinical conditions are ill-defined and can be misleading. This is a life-threatening condition and prompt management is essential to decrease the potential for thrombosis-related morbidity and mortality.
We report a case of internal jugular vein-sigmoid sinus thrombosis due to a misplaced central venous catheter.
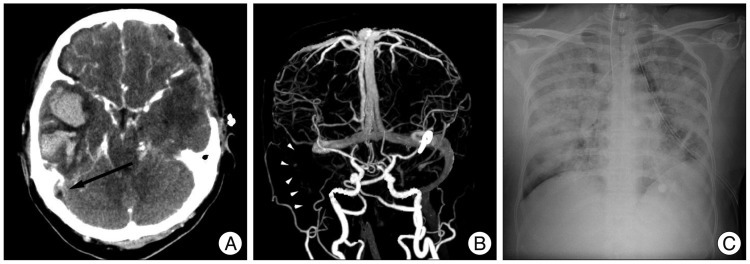
A 52-year-old woman presented with a severe bursting headache, vomiting, and a drowsy mentality. Brain computed tomography (CT) scan revealed subarachnoid hemorrhage in the basal cistern with a small amount of hematoma at the left Sylvian fissure (Fig. 1A). We identified an aneurysmal rupture of the middle cerebral artery on the left side by cerebral catheter angiography (Fig. 1B) and performed aneurysmal clipping. Postoperative chest X-ray was normal except for malposition of the central venous catheter in the right internal jugular vein (Fig. 1C). Unfortunately, we overlooked this important finding. Ten days later, the patient developed a fever, elevated white blood cell count to 227000, and elevated C-reactive protein to 33.6. The central venous catheter was removed, and antibiotics were administrated through a different intravenous route. Despite these managements, the patient was stupor the next day. We suspected vasospasm due to subarachnoid hemorrhage. Brain CT and CT angiography ruled out this possible diagnosis. Brain CT scan revealed cerebral infarction with hemorrhagic transformation in the right temporal lobe, and CT angiography did not identify vasospasm (Fig. 2A, B). Retrospective analysis of the contrast-enhanced CT scan and CT angiography showed an "empty delta sign" and absence of venous flow within the right internal jugular vein-sigmoid sinus that was sufficient for diagnosis of sinus thrombosis (Fig. 2A, B). Results of central venous catheter tip and blood culture was reported Staphylococcus epidermidis and Methicillin-resistant Staphylococcus aureus, respectively. Antibiotics were changed to vancomycin. We could not start systemic heparinization due to hemorrhagic transformation of the cerebral infarction in the right temporal lobe. We administrated mannitol and steroids to manage increased intracranial pressure; however, the patient died from severe pneumonia due to septic emboli after one week (Fig. 2C).
Central venous catheterization is commonly used procedure for the clinical management of many patients. Indications for a CVC are the intravenous administration of drugs, parenteral nutrition, hemodialysis, and hemodynamic monitoring13). At many institutions, CVC is routinely inserted before undergoing major surgery or treating patients with critical illnesses or cancer. The most frequently available anatomical sites for CVC are the subclavian and internal jugular veins. These procedures carry a substantial risk of mechanical lesions, such as arterial puncture, pneumothorax, cardiac tamponade, nerve lesions, or thrombotic or septic complications13).
Malpositioning of the catheter tip may happen more often in the subclavian vein than the internal jugular vein. The reported incidence of primary misplacement of the catheter tip after infraclavicular subclavian vein catheterization varies from 5% to 24% even when inserted by experienced clinicians7). Inadvertent catheterization of the ipsilateral internal jugular vein is one of the most common misplacements, with a reported incidence of around 7%15). Malpositioned catheters may lead to serious complications. The positioning of catheter tips within the cardiac silhouette is associated with increased risk of cardiac tamponade6). Also, positioning of the catheter tip in the subclavian vein is associated with a high risk of thrombus formation and vessel occlusion2). The risk of thrombosis may increase when hyperosmolar parenteral nutrition fluid is administered through a misplaced central venous catheter into a internal jugular vein3,15). Moreover, malpositioned catheter tips can damage the endothelium and precipitate the formation of thrombi17). When a CVC causes thrombosis, the risk of catheter-related sepsis may increase. In patients with a CVC, the risk of catheter-related infection was reported to range between 1% and 10%1). Contamination of a thrombus from the skin puncture site may result in septic endophlebitis, and occasional blood-borne infections may also contaminate the thrombus. Eventually, distant metastatic infections may develop. Embolic septic thrombi may involve the lungs and, less frequently, the joints, viscera, and brain17). This condition may increase the mortality of critically ill patients.
Subclavian or internal jugular vein thrombosis associated with indwelling catheters will propagate into other vessels, but extension into the intracranial sinuses and veins is rare. Three reports describe an association between cerebral venous sinus thrombosis and central venous hyperalimentation due to placement of the catheter tip in the internal jugular vein3,15,16). The authors warn of the potential for thrombosis due to retrograde infusion into the valveless internal jugular-dural sinus system but also suggest that the small caliber of the vein may predispose to thrombosis. In our case, CVC was performed to ready the patient to undergo surgery in the operating room. We found the malpositioned catheter tip in the internal jugular vein on follow-up chest X-ray, but it was ignored. All intravenous fluids (e.g., total parenteral nutrition, mannitol, antibiotics) were administered through the misplaced catheter. The patient's condition worsened, leading to thrombosis of the internal jugular vein secondary to sigmoid sinus thrombosis.
Since the advent of antibiotics, the incidence of septic sigmoid sinus thrombosis has significantly decreased. Fever, chills, otalgia, tenderness to percussion over the mastoid emissary vein, headache and vomiting are common but not pathognomonic features8,18). Occasionally, neurologic symptoms are related to increased intracranial pressure or infarct and present as deteriorating mental status, lethargy, seizures, hemiplegia, and coma, and may lead to death. Rarely, remote septic conditions such as pneumonia are the presenting symptoms9). Because of the nonspecific signs and symptoms of disease and the masking effects of antibiotics, diagnosis is difficult. Delays in diagnosis and treatment often result in high morbidity and mortality rates.
The diagnosis of sigmoid sinus thrombosis can be confirmed by CT, magnetic resonance imaging, or angiography. CT scan is the most widely used imaging method for finding intracranial lesions. Contrast-enhanced CT scans demonstrate multiple intraluminal filling defects and nonvisualization of the sinus18). In addition, low density lumen, sharply defined dense vessel wall, or distension of the thrombosed vein, such as the "empty delta sign", are positive signs for sinus thrombosis19). Vascular imaging with cerebral angiography is highly specific for the recognition of sinus thrombosis because it can detect the lack of blood flow in thrombosed cerebral veins and dural sinuses. Chest X-rays may also demonstrate septic embolic pleura-pulmonary complications, which are often bilateral, by revealing nodular infiltrates with pleural effusion17). Retrospectively, we confirmed that contrast-enhanced brain CT scans revealed a low density lumen surrounding a sharply enhanced dense vessel wall at the sigmoid sinus on the right side. Also, the right internal jugular vein and sigmoid sinus were not visualized on CT angiography. Finally, these septic emboli induced severe pneumonia.
Treatment consists of aggressive antimicrobial therapy, heparinization, anticoagulation, and decreasing intracranial pressure. Antibiotic selections is directed toward the causative pathogen cultured at the initial site of infection. Recent studies have shown heparinization to be safe and beneficial, despite the possibility of an increased risk of hemorrhage4,10,16). Currently, heparin is recommended as the initial drug of choice for cerebral venous sinus thrombosis followed by long-term anticoagulation with warfarin5,10,12). Anticoagulant therapy is recommended to reduce the risk of pulmonary embolism17). Chemical thrombolysis and mechanical thrombectomy have been described in patients refractory to anticoagulation. Although these procedures allow for rapid clot removal and reduction of venous hypertension, hemorrhagic complications can occur, leading to be high morbidity and mortality14). Occlusion of the cerebral veins due to thrombosis may induce localized brain edema and venous infarction resulting in elevated intracranial pressure. Finally, death frequently results from increased intracranial pressure caused by obstruction of venous and cerebrospinal outflow11).
In our case, we treated the patient empirically with antibiotics until bacteriologic results became available. Culture results of the subclavian catheter tip and blood were confirmed as Staphylococcus epidermidis and Methicillin-resistant Staphylococcus aureus, respectively, and we switched from broad-spectrum antibiotics to vancomycin. Unfortunately, we could not start heparinization due to concurrent cerebral hemorrhage of being transformed from cerebral infarction. Brain edema was slightly aggravated and we managed increased intracranial pressure. Severe pneumonia due to septic emboli eventually developed in both lung fields, and the patient died.
Diagnosis of internal jugular vein-sigmoid sinus thrombosis is challenging due to vague clinical features. Therefore, if patients with malpositioned CVCs present with symptoms of fever, chills, headache, vomiting, increased intracranial pressure, mental deterioration, and focal neurologic deficits, intracranial sinus thrombosis should be considered. Even vague symptoms may need to be investigated radiologically to discover a thrombosis early, when it can be treated. Therefore, careful study should be recommended for patients with confirmed intracranial sinus thrombosis.
References
1. Adal KA, Farr BM. Central venous catheter-related infections : a review. Nutrition. 1996; 12:208–213. PMID: 8798227.
2. Bennegård K, Curelaru I, Gustavsson B, Linder LE, Zachrisson BF. Material thrombogenicity in central venous catheterization. I. A comparison between uncoated and heparin-coated, long antebrachial, polyethylene catheters. Acta Anaesthesiol Scand. 1982; 26:112–120. PMID: 7048839.

3. Birdwell BG, Yeager R, Whitsett TL. Pseudotumor cerebri. A complication of catheter-induced subclavian vein thrombosis. Arch Intern Med. 1994; 154:808–811. PMID: 8147687.

4. Bousser MG. Cerebral venous thrombosis : diagnosis and management. J Neurol. 2000; 247:252–258. PMID: 10836615.
5. Bousser MG. Cerebral venous thrombosis : nothing, heparin, or local thrombolysis? Stroke. 1999; 30:481–483. PMID: 10066839.
6. Collier PE, Blocker SH, Graff DM, Doyle P. Cardiac tamponade from central venous catheters. Am J Surg. 1998; 176:212–214. PMID: 9737635.

7. Dunbar RD, Mitchell R, Lavine M. Aberrant locations of central venous catheters. Lancet. 1981; 1:711–715. PMID: 6110926.

8. Han YM, Lee JP, Hwang HS, Lim DC, Song JH, Ahn MS. Cerebral dural sinus thrombosis. J Korean Neurosurg Soc. 2001; 30:389–394.
9. Hawkins DB. Lateral sinus thrombosis : a sometimes unexpected diagnosis. Laryngoscope. 1985; 95:674–677. PMID: 3999902.
10. Kubiak BD, Albert SP, Tandoh MA, Fortune JB, Cunningham PR. Transverse sinus thrombosis after internal jugular vein ligation. J Emerg Med. 2012; 43:e5–e9. PMID: 19682827.

11. Larkey D, Williams CR, Fanning J, Hilgers RD, Graham DR, Fortin CJ. Fatal superior sagittal sinus thrombosis associated with internal jugular vein catheterization. Am J Obstet Gynecol. 1993; 169:1612–1614. PMID: 8267072.

12. Masuhr F, Einhäupl K. Treatment of cerebral venous and sinus thrombosis. Front Neurol Neurosci. 2008; 23:132–143. PMID: 18004059.

13. Ruesch S, Walder B, Tramèr MR. Complications of central venous catheters : internal jugular versus subclavian access--a systematic review. Crit Care Med. 2002; 30:454–460. PMID: 11889329.

14. Soleau SW, Schmidt R, Stevens S, Osborn A, MacDonald JD. Extensive experience with dural sinus thrombosis. Neurosurgery. 2003; 52:534–544. discussion 542-544. PMID: 12590677.

15. Souter RG, Mitchell A. Spreading cortical venous thrombosis due to infusion of hyperosmolar solution into the internal jugular vein. Br Med J (Clin Res Ed). 1982; 285:935–936.

16. Stephens PH, Lennox G, Hirsch N, Miller D. Superior sagittal sinus thrombosis after internal jugular vein cannulation. Br J Anaesth. 1991; 67:476–479. PMID: 1931408.

17. Tovi F, Fliss DM, Noyek AM. Septic internal jugular vein thrombosis. J Otolaryngol. 1993; 22:415–420. PMID: 8158736.
18. Tovi F, Hirsch M. Computed tomographic diagnosis of septic lateral sinus thrombosis. Ann Otol Rhinol Laryngol. 1991; 100:79–81. PMID: 1985532.

19. Zerhouni EA, Barth KH, Siegelman SS. Demonstration of venous thrombosis by computed tomography. AJR Am J Roentgenol. 1980; 134:753–758. PMID: 6767362.

Fig. 1
A 52-year-old woman suffered from a severe bursting headache, vomiting, and a drowsy mentality. Brain computed tomography scan (A) revealed subarachnoid hemorrhage in the basal cistern and a small hematoma at the left Sylvian fissure. On cerebral catheter angiography (B), we identified a saccular aneurysm at the bifurcation of the left middle cerebral artery (black arrow). Postoperative chest X-ray (C) shows malpositioning of the central venous catheter in the internal jugular vein (white arrow).
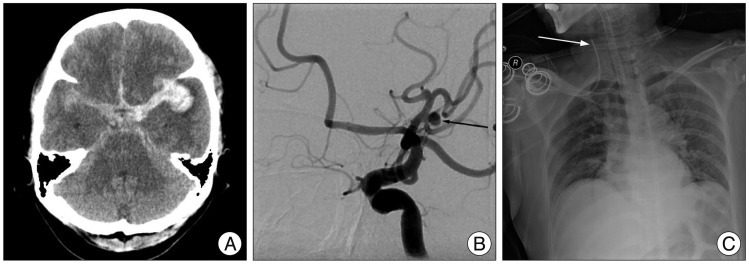
Fig. 2
Contrast-enhanced brain CT scan (A) reveals venous infarction with hemorrhagic transformation in the right temporal lobe. Retrospective analysis of the CT scan showed an "empty delta sign" (black arrow), sufficient for diagnosis of sigmoid sinus thrombosis. On anteroposterior view of CT angiography (B), right internal jugular vein and sigmoid sinus are not found due to absence of venous outflow (white arrow heads). Eventually, the patient died due to severe pneumonia from septic emboli (C).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download