Abstract
Objective
Transient anterograde amnesia is occasionally observed in a number of conditions, including migraine, focal ischemia, venous flow abnormalities, and after general anesthesia. The inhalation anesthetic, isoflurane, is known to induce transient anterograde amnesia. We examined the involvement of brain-derived neurotrophic factor (BDNF) and its receptor tyrosine kinase B (TrkB) in the underlying mechanisms of the isoflurane-induced transient anterograde amnesia.
Methods
Adult male Sprague-Dawley rats were divided into three groups : the control group, the 10 minutes after recovery from isoflurane anesthesia group, and the 2 hours after recovery from isoflurane anesthesia group (n=8 in each group). The rats in the isoflurane-exposed groups were anesthetized with 1.2% isoflurane in 75% nitrous oxide and 25% oxygen for 2 hours in a Plexiglas anesthetizing chamber. Short-term memory was determined using the step-down avoidance task. BDNF and TrkB expressions in the hippocampus were evaluated by immunofluorescence staining and western blot analysis.
Results
Latency in the step-down avoidance task was decreased 10 minutes after recovery from isoflurane anesthesia, whereas it recovered to the control level 2 hours after isoflurane anesthesia. The expressions of BDNF and TrkB in the hippocampus were decreased immediately after isoflurane anesthesia but were increased 2 hours after isoflurane anesthesia.
Anterograde amnesia is a syndrome that is characterized by a sudden onset of selective amnesia during which patients cannot store new memories after an event, while retrograde amnesia is a syndrome in which patients cannot recall old memories after an event, and these do not involve neurological deficits3,5,29). Transient anterograde amnesia is clinically dramatic but benign in nature. The hallmark of transient anterograde amnesia is brief inability to form new memories and recall past memories despite otherwise normal neurological function5,29). In a significant number of patients with transient anterograde amnesia, a stressful precipitating factor can be identified. Transient anterograde amnesia is occasionally observed in a number of conditions, including migraine, focal ischemia, venous flow abnormalities, and after general anesthesia5,29). Transient anterograde amnesia induces impairments in memory processes involving place, time, response, and perceptual and language information9).
The inhalation anesthetic, isoflurane, is halogenated ether that is used for general anesthesia. Isoflurane has many favorable effects, including analgesia, muscle relaxation, an absence of central nervous system (CNS) excitation, and neuroprotection. However, it occasionally induces problematic side effects, such as respiratory depression, reduced arterial blood pressure, amnesia, and unresponsiveness7,32,35). Of these several side effects, the amnesic action of isoflurane has received much interest, and many studies have been performed in order to evaluate its underlying mechanisms. Dutton et al.7) observed that isoflurane induced dose-dependent anterograde amnesia but not retrograde amnesia. Rau et al.26) suggested that γ-aminobutyric acid type (GABA) A receptor is the main causative factor of the amnestic effects of isoflurane on hippocampal-dependent declarative memory. The frequency of θ oscillations was also suggested as a parameter of isoflurane-induced anterograde amnesia24).
The hippocampus plays a key role in memory processes. Many neurotrophic factors have been suggested to be part of the underlying mechanisms associated with hippocampal-dependent memory. These neurotrophic factors include nerve growth factor, neurotrophin-3, neurotrophin-4/5, and brain-derived neurotrophic factor (BDNF). Among them, BDNF has been shown to have a crucial role as a modulator of long-term potentiation, synaptic plasticity, and neuronal plasticity in the adult CNS4,14,18). The tyrosine kinase B (TrkB) receptor is a specific receptor for BDNF, and it plays a key role in neuronal survival, differentiation, and synaptic plasticity6,23). Lu et al.15) reported that the levels of BDNF in the thalamus and cortex of rats were changed by exposure to general anesthesia. However, the effects of isoflurane on the expression of BDNF in the hippocampus in relation to anterograde amnesia are unknown.
In the present study, we investigated whether isoflurane anesthesia induces anterograde amnesia, and the underlying mechanisms of isoflurane-induced anterograde amnesia were evaluated in relation to the expressions of BDNF and TrkB in the rat hippocampus.
Adult male Sprague-Dawley rats weighing 250±10 g were used for this experiment. The animals were housed under conditions of controlled temperature (20±2℃) and lighting (07:00 to 19:00 hour), and food and water were supplied ad libitum. The experimental procedures progressed according to the animal care guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences. The rats were randomly divided into three groups : the control group, the 10 minutes after recovery from isoflurane anesthesia group, and the 2 hours after recovery from isoflurane anesthesia group (n=8 in each group). The rats in the isoflurane-exposed groups were anesthetized with 1.2% isoflurane in 75% nitrous oxide and 25% oxygen for 2 hours in a Plexiglas anesthetizing chamber.
In order to evaluate short-term memory loss, we conducted a step-down avoidance task according to the recovery time after isoflurane anesthesia, as previously described30). The rats were positioned on a 7×25 cm platform with a height of 2.5 cm, and they were then allowed to rest on the platform for 2 min. The platform faced a 42×25 cm grid of parallel 0.1-cm-caliber stainless steel bars, which were spaced 1 cm apart. In the training session, the animals received a 0.5-mA scramble foot shock for 20 seconds immediately upon stepping down. In the control group, the training session was conducted 2 hours after rest and without isoflurane anesthesia. The training session in the other groups was conducted according to the recovery time of the respective group (10 minutes or 2 hours after isoflurane anesthesia). The retention time in each group was assessed 10 minutes after the training session of each group. The interval of time in which the rats stepped down and placed all four paws on the grid was defined as the latency of the step-down avoidance task. Any latency >300 seconds was counted as 300 sec.
After the determination of the retention time in the step-down avoidance task, the rats were deeply anesthetized with Zoletil 50® anesthesia (10 mg/kg, i.p.; Virbac, Carros, France). For immunofluorescence, the rats were transcardially perfused with 50 mM phosphate-buffered saline (PBS), which was followed by 4% paraformaldehyde in 0.5 M sodium phosphate buffer at pH 7.4. The brains were removed, postfixed in the same fixative overnight, and transferred to a 30% sucrose solution for cryoprotection. Serial coronal sections that were 40 µm thick were cut with a freezing microtome (Leica Biosystems Nussloch GmbH, Nussloch, Germany).
Western blot analysis was conducted as previously described method11). For western blotting, the hippocampus was removed from each rat brain, and the extraneous tissue was trimmed away. The hippocampal tissues were minced and chopped in lysate buffer that contained 50 mM HEPES (pH 7.5), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM magnesium chloride hexahydrate, 1 mM ethyleneglycol-bis-(β-aminoethyl ether)-N,N'-tetraacetic acid, 1 mM phenylmethylsulfonyl fluoride, 2 µg/mL leupeptin, 1 µg/mL pepstatin, 1 mM sodium orthovanadate, and 100 mM sodium fluoride. After homogenization, the tissues were reacted with lysate buffer for 20 min and then centrifuged at 14000 rpm for 20 minutes at 4℃. The supernatant was stored in a -70℃ deep freezer.
Whole protein extracts were used to evaluate the levels of expression of the BDNF and TrkB proteins. The protein concentrations were measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). Forty µg of protein was separated on SDS-polyacrylamide gels and then transferred onto a nitrocellulose membrane (Schleicher & Schuell Bioscience GmbH, Dassel, Germany). A rabbit BDNF antibody (1 : 1000; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and a rabbit TrkB antibody (1 : 1000; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) were used as the primary antibodies. An anti-rabbit antibody (1 : 2000; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) was used as the secondary antibody for BDNF and TrkB. Bands were detected using the enhanced chemiluminescence detection system (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA).
For the detection of intensities of BDNF and TrkB in the hippocampal CA3 region, immunofluorescence staining was performed. After washing the free-floating sections 3 times using 50 mM PBS, we incubated the sections in 3% H2O2 for 30 minutes in order to block endogenous peroxidase activity. Next, the sections were incubated in blocking solution (1% bovine serum albumin and 10% goat serum in 0.05 M PBS) for 2 hours at room temperature and then incubated overnight with rabbit BDNF antibody (1 : 200; Santa Cruz Biotechnology Inc.) or rabbit TrkB antibody (1 : 200; Santa Cruz Biotechnology Inc.). In order to verify the precise BDNF and TrkB expression, we performed counterstaining on the same sections using a mouse anti-neuronal nuclei antibody (1 : 200; Millipore, Billerica, MA, USA). The sections were next incubated for 2 hours with a FITC-conjugated goat anti-rabbit secondary antibody (Vector Laboratories Inc., Burlingame, CA, USA) and a FITC-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA). The sections were then mounted on gelatin-coated glass slides, and the coverslips were mounted using fluorescent mounting medium (Dako North America Inc., Carpinteria, CA, USA). The fluorescent images were captured using confocal laser-scanning microscopy with LSM 510 META (Carl Zeiss MicroImaging GmbH, Oberkochen, Germany). Negative controls were performed by omitting the primary antibodies, and these sections did not show any signals.
In order to compare the relative levels of expression of BDNF and TrkB, detected bands for western blot and intensity for immunofluorescence were evaluated densitometrically using an Image-Pro® computer-assisted image analysis system (Media Cybernetics Inc., Silver Spring, MD, USA). The results were expressed as the mean±standard error of the mean. All data were analyzed by one-way ANOVA, which was followed by Duncan's posthoc test using SPSS (IBM Corporation, Chicago, IL, USA). p values less than 0.05 were considered statistically significant.
The latency in the step-down avoidance task was 295.87±2.17 seconds in the control group, 10.25±2.19 seconds in the 10 minutes after recovery from isoflurane anesthesia group, and 289.25±5.72 seconds in the 2 hours after recovery from isoflurane anesthesia group (Fig. 1). The latency was significantly decreased 10 minutes after recovery from isoflurane anesthesia (p<0.05). However, latency recovered to the control level 2 hours after isoflurane anesthesia (p<0.05).
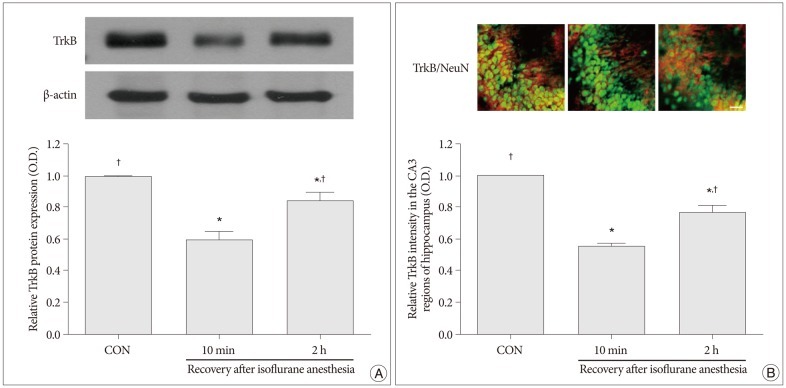
Western blot analyses for the protein levels of BDNF and TrkB in the hippocampus were performed in order to provide relative levels of expressions of these proteins. In the present study, the protein levels of BDNF and TrkB in the control group were set as 1.00. The relative levels of expression of BDNF were 0.52±0.06 in the 10 minutes after recovery from isoflurane anesthesia group and 0.78±0.01 in the 2 hours after recovery from isoflurane anesthesia group (p<0.05) (Fig. 2A). The relative levels of expression of TrkB was 0.59±0.05 in the 10 minutes after recovery from isoflurane anesthesia group and 0.85±0.04 in the 2 hours after recovery from isoflurane anesthesia group (p<0.05) (Fig. 3A). The protein levels of BDNF and TrkB were significantly decreased in the 10 minutes after isoflurane anesthesia group. However, these protein levels were increased in the 2 hours after isoflurane anesthesia group.
Immunofluorescent photomicrographs of BDNF and TrkB expressions in the hippocampal CA3 region are presented in Fig. 2B and Fig. 3B. In the present study, the intensity levels of BDNF and TrkB in the control group were set as 1.00. The relative intensity levels of BDNF were 0.55±0.04 in the 10 minutes after recovery from isoflurane anesthesia group and 0.74±0.02 in the 2 hours after recovery from isoflurane anesthesia group (p<0.05) (Fig. 2B). The relative intensity levels of TrkB were 0.55±0.03 in the 10 min after recovery from isoflurane anesthesia group and 0.76±0.04 in the 2 h after recovery from isoflurane anesthesia group (p<0.05) (Fig. 3B). The expressions of BDNF and TrkB in the hippocampcal CA3 region were decreased in the 10 minutes after recovery from isoflurane anesthesia group compared to the control group. However, the expressions of BDNF and TrkB in the hippocampcal CA3 region recovered to near control values in the 2 hours after recovery from isoflurane anesthesia group.
Transient anterograde amnesia is one of the key desirable endpoints of general anesthesia. After surgery, the recovered patients can respond to doctors' requests or to stimulations that evaluate neurologic symptoms or muscle relaxation reversal. However, most of the patients do not remember these checks. Because the presentation of transient anterograde amnesia can be dramatic and may mimic an acute cerebral ischemic event, a thorough neurologic evaluation should be pursued. Transient anterograde amnesia is characterized by a sudden memory loss of recent and/or remote events and transient inability to acquire new knowledge. The dramatic presentation of acute change in personality, after emergence from general anesthesia, creates a puzzling and frightening situation for both the patient's family and the anesthesiologist5).
Twersky et al.31) reported that midazolam provided partial anterograde amnesia in pediatric patients. In addition, Münte et al.22) demonstrated that balanced anesthesia techniques with isoflurane or propofol led to minimal implicit memory in patients undergoing lumbar disc surgery. The amnestic effects of isoflurane have been reported in many studies24,26,34). However, the exact mechanism by which the inhaled anesthetic isoflurane produces amnesia is not understood. Previous studies demonstrated that GABAA receptor is implicated in the anxiolytic, amnesic, and sedative behavioral effects of volatile anesthetics and alcohol8,26,34). Perouansky et al.24) reported that halothane, nitrous oxide, and isoflurane slowed θ peak frequencies, suggesting that modulation of the θ rhythm contributes to the anesthetic-induced amnesia.
In the present study, we first evaluated whether isoflurane anesthesia induces transient anterograde amnesia using a step-down avoidance task. We executed the step-down avoidance task in order to determine the latency according to the time after recovery from anesthesia (10 minutes or 2 hours). In the present results, the latency was shortened 10 minutes after recovery from isoflurane anesthesia, and the latency was recovered to control level 2 hours after recovery from isoflurane anesthesia. These results indicate that temporal memory loss was induced by isoflurane anesthesia, and the memory loss disappeared 2 hours later, suggesting that isoflurane anesthesia induced transient anterograde amnesia. Transient anterograde amnesia has two types of amnesia : global type and focal (localized) type. In the present study, the rats showed transient memory loss on the electric shock 10 minutes after recovery from isoflurane anesthesia, this type of amnesia might be considered as the focal type of amnesia.
We next investigated whether the expressions of BDNF and its receptor TrkB in the hippocampus were related to the transient anterograde amnesia. BDNF enhances synaptic transmission and neuronal plasticity in the CNS28), resulting in increase of learning abilities and memory capabilities20). BDNF is released from synapses in an activity-dependent manner and acts on postsynaptic neurons12). This action of BDNF is involved in the representing, processing, and storing information in the complex neural networks2,16,33). Because TrkB is a high-affinity receptor for BDNF, abnormal TrkB expression impairs hippocampal-dependent memory and learning tasks19,27). Released BDNF strengthens individual synapses through its receptor TrkB in the hippocampus. BDNF influences cell survival or apoptosis through the activation of TrkB14,16). Overexpression of BDNF and its receptor TrkB improves learning or cognitive behavior, whereas the inhibition of BDNF or TrkB causes memory impairment1,13,17,19,21,25). BDNF was also suggested as the main factor in the maternal exercise-induced enhancement of memory in rat pups10).
In the present results, western blotting showed that the expressions of BDNF and TrkB in the hippocampus were significantly decreased 10 minutes after recovery from isoflurane anesthesia. However, 2 hours after recovery from isoflurane anesthesia, the expressions of BDNF and TrkB in the hippocampus were recovered near to the control level. Immunofluorescence staining also revealed that the intensities of BDNF and TrkB in the hippocampal CA3 region were significantly decreased 10 minutes after recovery from isoflurane anesthesia. However, 2 hours after recovery from isoflurane anesthesia, the intensities of BDNF and TrkB in the hippocampal CA3 region were recovered near to the control level. These results indicate that isoflurane anesthesia suppressed the expressions of BDNF and TrkB in the hippocampus. However, this suppression disappeared 2 hours later, suggesting that the expressions of BDNF and TrkB were closely linked with isoflurane-induced transient anterograde amnesia in rats.
In the present results, temporary memory loss was induced by isoflurane anesthesia, and this memory loss disappeared 2 hours later. The expressions of BDNF and TrkB in the hippocampus were decreased immediately after isoflurane anesthesia but were increased 2 hours after isoflurane anesthesia. We showed that isoflurane anesthesia induced transient anterograde amnesia and that the levels of BDNF and TrkB in the hippocampus might be involved in the underlying mechanism of this transient anterograde amnesia.
Acknowledgements
This research was supported by the Research Fund from Kyung Hee University at the year of 2009 (KHU 20090569).
References
1. Alonso M, Vianna MR, Depino AM, Mello e Souza T, Pereira P, Szapiro G, et al. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002; 12:551–560. PMID: 12201640.

2. Arancio O, Chao MV. Neurotrophins, synaptic plasticity and dementia. Curr Opin Neurobiol. 2007; 17:325–330. PMID: 17419049.

3. Bartsch T, Schönfeld R, Müller FJ, Alfke K, Leplow B, Aldenhoff J, et al. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science. 2010; 328:1412–1415. PMID: 20538952.

4. Bliss TV, Collingridge GL. A synaptic model of memory : long-term potentiation in the hippocampus. Nature. 1993; 361:31–39. PMID: 8421494.

5. Bortolon RJ, Weglinski MR, Sprung J. Transient global amnesia after general anesthesia. Anesth Analg. 2005; 101:916–919. PMID: 16116014.

6. Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci. 2010; 3:1. PMID: 20162032.

7. Dutton RC, Maurer AJ, Sonner JM, Fanselow MS, Laster MJ, Eger EI 2nd. Isoflurane causes anterograde but not retrograde amnesia for pavlovian fear conditioning. Anesthesiology. 2002; 96:1223–1229. PMID: 11981164.

8. Jia F, Pignataro L, Harrison NL. GABAA receptors in the thalamus : alpha4 subunit expression and alcohol sensitivity. Alcohol. 2007; 41:177–185. PMID: 17521848.

9. Kesner RP, Goodrich-Hunsaker NJ. Developing an animal model of human amnesia : the role of the hippocampus. Neuropsychologia. 2010; 48:2290–2302. PMID: 19883669.

10. Kim H, Lee SH, Kim SS, Yoo JH, Kim CJ. The influence of maternal treadmill running during pregnancy on short-term memory and hippocampal cell survival in rat pups. Int J Dev Neurosci. 2007; 25:243–249. PMID: 17434282.

11. Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, et al. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010; 45:357–365. PMID: 20156544.

12. Kohara K, Kitamura A, Morishima M, Tsumoto T. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science. 2001; 291:2419–2423. PMID: 11264540.

13. Koponen E, Võikar V, Riekki R, Saarelainen T, Rauramaa T, Rauvala H, et al. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLCgamma pathway, reduced anxiety, and facilitated learning. Mol Cell Neurosci. 2004; 26:166–181. PMID: 15121188.

14. Lee E, Son H. Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep. 2009; 42:239–244. PMID: 19470236.

15. Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V. General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis. 2006; 11:1603–1615. PMID: 16738805.

16. Lu Y, Christian K, Lu B. BDNF : a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008; 89:312–323. PMID: 17942328.

17. Ma YL, Wang HL, Wu HC, Wei CL, Lee EH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998; 82:957–967. PMID: 9466420.

18. Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory : an evaluation of the hypothesis. Annu Rev Neurosci. 2000; 23:649–711. PMID: 10845078.
19. Minichiello L, Korte M, Wolfer D, Kühn R, Unsicker K, Cestari V, et al. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999; 24:401–414. PMID: 10571233.

20. Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000; 20:7116–7121. PMID: 10995859.

21. Mu JS, Li WP, Yao ZB, Zhou XF. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999; 835:259–265. PMID: 10415381.

22. Munte S, Schmidt M, Meyer M, Nager W, Lüllwitz E, Münte TF, et al. Implicit memory for words played during isoflurane- or propofol-based anesthesia : the lexical decision task. Anesthesiology. 2002; 96:588–594. PMID: 11873032.
23. Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol. 2001; 63:71–124. PMID: 11040419.

24. Perouansky M, Rau V, Ford T, Oh SI, Perkins M, Eger EI 2nd, et al. Slowing of the hippocampal θ rhythm correlates with anesthetic-induced amnesia. Anesthesiology. 2010; 113:1299–1309. PMID: 21042201.

25. Pietropaolo S, Paterna JC, Büeler H, Feldon J, Yee BK. Bidirectional changes in water-maze learning following recombinant adenovirus-associated viral vector (rAAV)-mediated brain-derived neurotrophic factor expression in the rat hippocampus. Behav Pharmacol. 2007; 18:533–547. PMID: 17762522.

26. Rau V, Iyer SV, Oh I, Chandra D, Harrison N, Eger EI 2nd, et al. Gamma-aminobutyric acid type A receptor alpha 4 subunit knockout mice are resistant to the amnestic effect of isoflurane. Anesth Analg. 2009; 109:1816–1822. PMID: 19923508.

27. Saarelainen T, Pussinen R, Koponen E, Alhonen L, Wong G, Sirviö J, et al. Transgenic mice overexpressing truncated trkB neurotrophin receptors in neurons have impaired long-term spatial memory but normal hippocampal LTP. Synapse. 2000; 38:102–104. PMID: 10941145.

28. Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000; 23:639–645. PMID: 11137155.

29. Shekhar R. Transient global amnesia--a review. Int J Clin Pract. 2008; 62:939–942. PMID: 18248396.
30. Sung YH, Shin MS, Cho S, Baik HH, Jin BK, Chang HK, et al. Depression-like state in maternal rats induced by repeated separation of pups is accompanied by a decrease of cell proliferation and an increase of apoptosis in the hippocampus. Neurosci Lett. 2010; 470:86–90. PMID: 20043974.

31. Twersky RS, Hartung J, Berger BJ, McClain J, Beaton C. Midazolam enhances anterograde but not retrograde amnesia in pediatric patients. Anesthesiology. 1993; 78:51–55. PMID: 8424571.

32. Wade JG, Stevens WC. Isoflurane: an anesthetic for the eighties? Anesth Analg. 1981; 60:666–682. PMID: 7023281.
33. Waterhouse EG, Xu B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol Cell Neurosci. 2009; 42:81–89. PMID: 19577647.

34. Werner DF, Swihart A, Rau V, Jia F, Borghese CM, McCracken ML, et al. Inhaled anesthetic responses of recombinant receptors and knockin mice harboring α2(S270H/L277A) GABA(A) receptor subunits that are resistant to isoflurane. J Pharmacol Exp Ther. 2011; 336:134–144. PMID: 20807777.

35. Yu Q, Wang H, Chen J, Gao Y, Liang W. Neuroprotections and mechanisms of inhalational anesthetics against brain ischemia. Front Biosci (Elite Ed). 2010; 2:1275–1298. PMID: 20515801.
Fig. 1
Latency in the step-down avoidance task. Latency was expressed as the interval of time in which rats stepped down and placed all four paws on the grid. Any latency >300 seconds was counted as 300 seconds. Results are presented as the mean±standard error of the mean. CON : control group, 10 min : 10 minutes after recovery from isoflurane anesthesia group, 2 h : 2 hours after recovery from isoflurane anesthesia group. *p<0.05 compared to the control group, †p<0.05 compared to the 10 minutes after recovery from isoflurane anesthesia group.
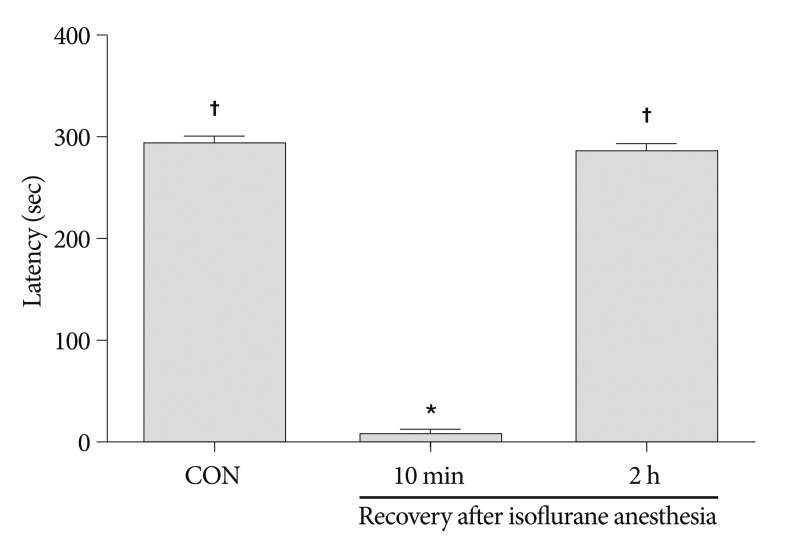
Fig. 2
Expression of brain-derived neurotrophic factor (BDNF) in the hippocampus. A : Levels of BDNF expression in the hippocampus as shown by western blot and quantitative analyses. B : Photomicrographs showing the neuronal nuclei (NeuN) and BDNF-specific double immunofluorescence staining in CA3 region of the hippocampus. The scale bar represents 100 µm. BDNF expression in the hippocampus of the control group was set as 1.00. The results are presented as the mean±standard error of the mean. CON : control group, 10 min : 10 minutes after recovery from isoflurane anesthesia group, 2 h : 2 hours after recovery from isoflurane anesthesia group, *p<0.05 compared to the control group, †p<0.05 compared to the 10 minutes after recovery from isoflurane anesthesia group.
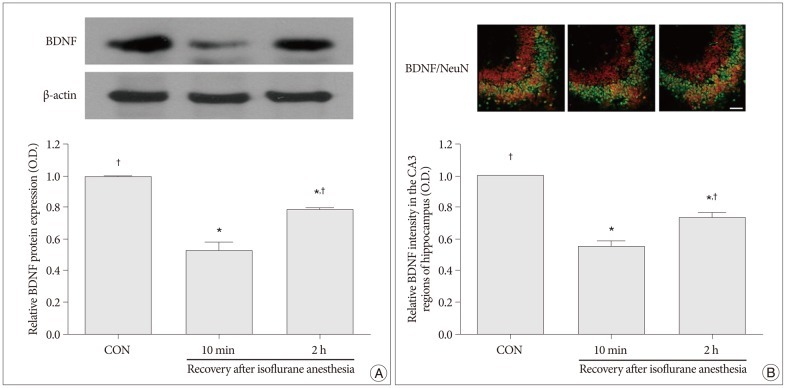
Fig. 3
Expression of tyrosine kinase B (TrkB) in the hippocampus. A : Levels of TrkB expression in the hippocampus as shown by western blot and quantitative analyses. B : Photomicrographs showing the neuronal nuclei (NeuN) and TrkB-specific double immunofluorescence staining in CA3 region of the hippocampus. The scale bar represents 50 µm. Levels of TrkB expression in the hippocampus in the control group were set as 1.00. The results are presented as the mean±standard error of the mean. CON : control group, 10 min : 10 minutes after recovery from isoflurane anesthesia group, 2 h : 2 hours after recovery from isoflurane anesthesia group. *p<0.05 compared to the control group, †p<0.05 compared to the 10 minutes after recovery from isoflurane anesthesia group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download