Abstract
Alternative to carotid endarterectomy, carotid artery stenting (CAS) can be performed for symptomatic severe stenosis of internal carotid artery, especially for high-risk patients. Among several complications after CAS, subacute in-stent thrombosis is rare but important, because patient's condition can deteriorate rapidly. Subacute in-stent thrombosis with carotid artery occlusion can be managed by superficial temporal artery-middle cerebral artery (STA-MCA) anastomosis. We report two cases of STA-MCA anastomosis for internal carotid artery occlusion by subacute in-stent thrombosis after CAS.
Carotid artery stenting (CAS) is an alternative option to carotid endarterectomy in patients with symptomatic severe stenosis of internal carotid artery (ICA), especially in high risk surgical candidates2,3). After CAS, in-stent thrombosis with ICA occlusion may occur shortly after hospital discharge. Superficial temporal artery-middle cerebral artery (STA-MCA) anastomosis was performed in two cases.
A 69-year-old male patient visited the emergency department due to dysarthria and left extremity weakness. The National Institutes of Health Stroke Scale (NIHSS) score was 3 points. Brain MRI showed small brain stem infarction on diffusion weighted image (Fig. 1A). Cerebral angiography revealed severe stenosis (83% in diameter stenosis) at right proximal ICA (Fig. 1B). Right CAS and balloon angioplasty were performed and there were no perioperative problems (Fig. 1C). The patient was discharged with dual antiplatelet medication therapy. However, nine days after CAS, the patient revisited the emergency department because of hemiparesis, dysarthria, and left facial palsy. The patient stated that the symptoms had been gradually aggravated since three days prior to the visit. NIHSS score was 8 points. In cerebral angiography, right proximal ICA occlusion was identified (Fig. 1D). In brain MRI, hemodynamic cerebral infarction was noted on diffusion image and diffusion-perfusion mismatch was seen on perfusion image (Fig. 1E, F). Thirteen days after the occurrence of right proximal ICA occlusion, STA-MCA anastomosis was performed. His symptoms were improved (Fig. 1G), but left hemiparesis and dysarthria were remained. Three months after surgery, his mRS score was 3 points.
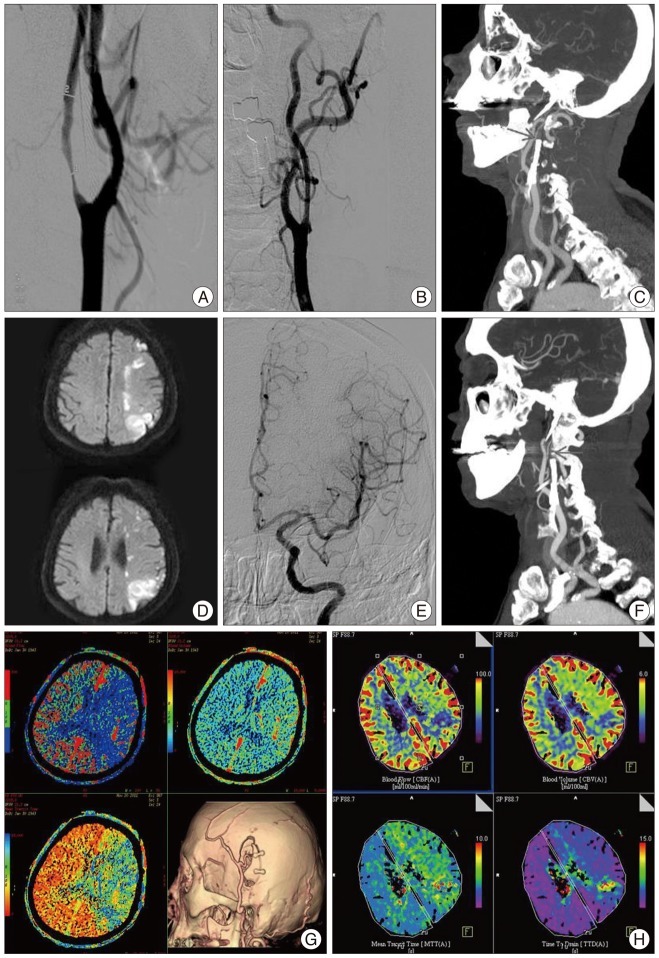
A 68-year-old male patient was admitted to the hospital with asymptomatic bilateral carotid stenosis. Severe stenosis at the right proximal ICA (94%) and stenosis at the left proximal ICA (34%) were identified in cerebral angiography. Right CAS and balloon angioplasty were performed. After 5 month, aggravated left proximal ICA stenosis (72%) was found during the follow-up (Fig. 2A). Left CAS and balloon angioplasty were also performed (Fig. 2B). He was discharged after starting dual antiplatelet medication. Four days after CAS, sudden right hemiparesis, dysarthria, and right facial palsy were noted. The patient was brought to the emergency department. NIHSS score at the admission was 2 points and NIHSS score in 6 hours from onset of symptom was 16 points which indicated that the symptoms were progressed. Proximal ICA occlusion was identified in neck angio CT (Fig. 2C). Intravenous tissue plasminogen activator and Intra-arterial thrombolysis were failed (Fig. 2D-F). Diffusion-perfusion mismatch was seen on perfusion CT image. Therefore, urgent STA-MCA anastomosis was performed (Fig. 2G, H). Aspirin and clopidogrel resistance test showed that the patient was resistant to both drugs. Triple antiplatelet medication therapy was started. The symptoms improved, but right hemiparesis was remained. Three months after the surgery, his mRS score was 3 points.
In-stent thrombosis is a stressful situation to doctors and is also a life-threatening moment to patients. Prompt diagnosis and reperfusion is vital to limit cerebral ischemia10). However, the incidence, pathophysiology and treatment of subacute in-stent thrombosis have hardly been reported in current interventional neurology literature to date. Therefore, the authors consulted interventional cardiology literature. Cardiologists defined subacute thrombosis as occlusion at the stented site within the first 28 days after the procedure12). Several theories have been suggested to explain the reasons for in-stent thrombosis. A low response or non-response to platelet inhibitors (e.g., aspirin or clopidogrel), atherosclerotic stenosis plaque rupture with the release of intrinsic factors promoting thrombosis in patients, inappropriate pretreatments, dissection at the uncovered stent portion, and a high degree of residual stenosis are included in these theories6). The rate of in-stent thrombosis is known to be seven times higher in intracranial stenting than coronary artery stenting. This might be due to the difference in the size of vessel lumen or perfusion pressure, incognizance of some subacute coronary stent thrombosis, and specific differences in the vessel wall regarding platelet adherence12). Van Laanen et al.13) suggested advanced age, female gender, implantation of multiple stents, prior revascularization treatment, suboptimal result with residual stenosis, elevated postprocedural serum levels of acute-phase reactants, asymptomatic lesion, and use of balloon expandable stents as the predictors of in-stent restenosis after CAS. Watarai et al.14) reported that the occurrence rate of acute or subacute in-stent thrombosis among 23 cases of CAS was 43.5% and the problem was mostly resolved within 12 weeks owing to endogenous thrombolysis. They also reported lengths of stenosis and stent lengths as the risk factors of in-stent thrombosis. There was a relatively high prevalence of antiplatelet drug resistance in patients with atherosclerotic cerebrovascular disease12). Furthermore, acute in-stent thrombosis was more frequently seen in the antiplatelet drug resistant group11). Although in-stent thrombosis do not always occur in antiplatelet drug resistant patients, it is thought to be related10,12). Feher et al.4) reported that aspirin/clopidogrel resistance seemed to be associated with poor clinical outcomes. In neurointervention, the guideline of medications has not yet been clearly established. However, antiplatelet drug resistance tests and the adjustment of antiplatelet drug medication are widely accepted and used. In addition to the conventional dual antiplatelet drug therapy (aspirin 100 mg+clopidogrel 75 mg), an increase of clopidogrel dosage or an addition of other medicine might be necessary.
Carotid artery occlusion may occur after carotid artery stenting. In our cases, uncontrollable hemodynamic infarction was occured. STA-MCA anastomosis was performed. After intensive care, the patient conditions were stabilized but neurologic sequelae were remained. These results implied a benefit of revascularization surgery.
According to the American Stroke Association Guideline for the Early Management of Adults With Ischemic Stroke, EC-IC bypass did not help patients with acute cerebral infarction because of intracranial hemorrhage1). However, the guideline introduced the favorable results with emergency bypass procedures9,15). Owing to the recent development of diagnostic techniques, the mechanisms of symptom development or fluctuation have been understood and this has helped the selection of candidates who likely to benefit from urgent reperfusion procedures. In fact, research results regarding this have been published. A JET study reported that EC-IC bypass surgery reduced the incidence of major stroke or death in the 2 years after the surgery8). Jeffree and Stoodley7) reported that the stroke incident rate after bypass surgery was 8% per year among patients with symptomatic carotid occlusion and the incident rate was 18% per year among patients who were waiting for the surgery. Hwang et al.5) described STA-MCA anastomosis as low flow bypass and reported that it had no complications associated with reperfusion compared with high flow bypass. Therefore, STA-MCA anastomosis may be beneficial to patients with acute ischemic stroke or stroke in progress in whom intra-arterial thrombolysis is not possible.
References
1. Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke : a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups : the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007; 38:1655–1711. PMID: 17431204.

2. Brott TG, Hobson RW 2nd, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010; 363:11–23. PMID: 20505173.

3. Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study) : an interim analysis of a randomised controlled trial. Lancet. 2010; 375:985–997. PMID: 20189239.
4. Feher G, Feher A, Pusch G, Koltai K, Tibold A, Gasztonyi B, et al. Clinical importance of aspirin and clopidogrel resistance. World J Cardiol. 2010; 2:171–186. PMID: 21160749.

5. Hwang G, Oh CW, Bang JS, Jung CK, Kwon OK, Kim JE, et al. Superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke and stroke in progress. Neurosurgery. 2011; 68:723–729. discussion 729-730. PMID: 21311299.

6. Iancu A, Grosz C, Lazar A. Acute carotid stent thrombosis : review of the literature and long-term follow-up. Cardiovasc Revasc Med. 2010; 11:110–113. PMID: 20347802.

7. Jeffree RL, Stoodley MA. STA-MCA bypass for symptomatic carotid occlusion and haemodynamic impairment. J Clin Neurosci. 2009; 16:226–235. PMID: 19097907.

8. JET Study Group. [Japanese EC-IC Bypass Trial (JET study) : study design and interim analysis]. Surg Cereb Stroke. 2002; 30:97–100.
9. Kakinuma K, Ezuka I, Takai N, Yamamoto K, Sasaki O. The simple indicator for revascularization of acute middle cerebral artery occlusion using angiogram and ultra-early embolectomy. Surg Neurol. 1999; 51:332–341. PMID: 10086500.

10. Lee CY, Ryu CW, Koh JS, Kim EJ. Total occlusion of the internal carotid artery by subacute in-stent thrombosis and subsequent spontaneous recanalization after stent-assisted coil embolization. Neurointervention. 2011; 6:38–41. PMID: 22125748.

11. Rho GJ, Shin WR, Kong TS, Kim MS, Lee CJ, Lee BH. Significance of clopidogrel resistance related to the stent-assisted angioplasty in patients with atherosclerotic cerebrovascular disease. J Korean Neurosurg Soc. 2011; 50:40–44. PMID: 21892403.

12. Riedel CH, Tietke M, Alfke K, Stingele R, Jansen O. Subacute stent thrombosis in intracranial stenting. Stroke. 2009; 40:1310–1314. PMID: 19213948.

13. Van Laanen J, Hendriks JM, Van Sambeek MR. Factors influencing restenosis after carotid artery stenting. J Cardiovasc Surg (Torino). 2008; 49:743–747.
14. Watarai H, Kaku Y, Yamada M, Kokuzawa J, Tanaka T, Andoh T, et al. Follow-up study on in-stent thrombosis after carotid stenting using multidetector CT angiography. Neuroradiology. 2009; 51:243–251. PMID: 19172256.

15. Yoshimoto Y, Kwak S. Superficial temporal artery--middle cerebral artery anastomosis for acute cerebral ischemia : the effect of small augmentation of blood flow. Acta Neurochir (Wien). 1995; 137:128–137. discussion 137. PMID: 8789652.

Fig. 1
A : Brain magnetic resonance imaging (MRI) shows small brain stem infarction on diffusion weighted image. B : Cerebral angiography reveals severe stenosis (83%) at right proximal internal carotid artery (ICA). C : Right carotid artery stenting and balloon angioplasty is being performed. D : In cerebral angiography, right proximal ICA occlusion is seen. E : In brain MRI, hemodynamic cerebral infarction is noted on diffusion image. F : Perfusion CT, diffusion-perfusion mismatch is seen. G : post-operation 7 days, follow-up computed tomography (CT) angiography and perfusion CT reveals maintenance of bypass flow and improvement of perfusion.
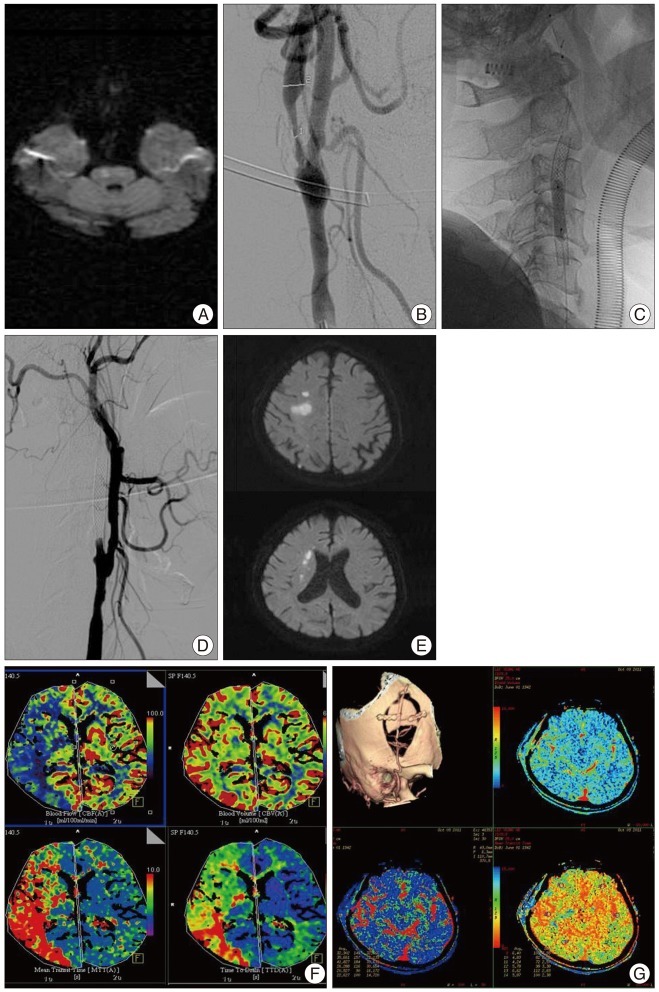
Fig. 2
A : In cerebral angiography, left proximal internal carotid artery (ICA) stenosis (72%) is aggravated. B : Left carotid artery stenting (CAS) and balloon angioplasty are done. C : Left proximal ICA occlusion is observed in neck angio CT (arrow). D : In brain MRI, hemodynamic cerebral infarction is noted on diffusion image.(Hemodynamic cerebral infarction is observed on diffusion view of brain MRI). E : After intravenous tissue plasminogen activator, angiographic revascularization of left ICA territory is confirmed through intra-arterial thrombolysis. F : Neck angio CT is performed since the patient's symptoms are not improved even after the intra-arterial and left proximal ICA occlusion is identified again (arrow). G : Diffusion-perfusion mismatch is seen on pre-operation perfusion CT image. Follow-up angio CT after the operation shows well-maintained bypass flow. H : Post-operation seven days, follow-up perfusion CT reveals improvement of cerebral blood flow.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download