Abstract
Objective
We compared the survival time between patients with multiple gamma knife radiosurgery (GKRS) and patients with a single GKRS plus whole brain radiation therapy (WBRT), in patients with multiple metachronous brain metastases from lung cancer.
Methods
From May 2006 to July 2010, we analyzed 31 patients out of 112 patients who showed multiple metachronous brain metastases. 20 out of 31 patients underwent multiple GKRS (group A) and 11 patients underwent a single GKRS plus WBRT (group B). We compared the survival time between group A and B. Kaplan-Meier method and Cox proportional hazards were used to analyze relationship between survival and 1) the number of lesions in each patient, 2) the average volume of lesions in each patient, 3) the number of repeated GKRS, and 4) the interval of development of new lesions, respectively.
Results
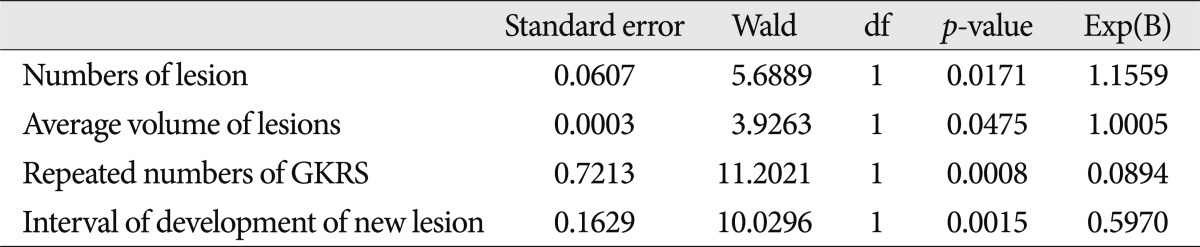
Median survival time was 18 months (range 6-50 months) in group A and 6 months (range 3-18 months) in group B. Only the average volume of individual lesion (over 10 cc) was negatively related with survival time according to Kaplan-Meier method. Cox-proportional hazard ratio of each variable was 1.1559 for the number of lesions, 1.0005 for the average volume of lesions, 0.0894 for the numbers of repeated GKRS, and 0.5970 for the interval of development of new lesions.
Conclusion
This study showed extended survival time in group A compared with group B. Our result supports that multiple GKRS is of value in extending the survival time in patients with multiple metachronous brain metastases, and that the number of the lesions and the frequency of development of new lesions are not an obstacle in treating patients with GKRS.
Brain metastases presenting as multiple lesions (more than three in number) correlate with a poor median survival time of 3-6 months4). Wong et al.20) reported a median survival time of 5.8 months in patients with solitary brain metastases and 3.5 months of median survival time in those with multiple metastases. However, Karlsson et al.13) found patients with controlled primary diseases who underwent gamma knife radiosurgery (GKRS) had median survival times of 12.1 months for single lesions and 8.6 months for multiple lesions. Bhatnagar et al.5) showed all patients with four or more intracranial metastases had a median overall survival time of 8 months after radiosurgery. Chang et al.6) reported that their patients showed the median survival time of 10 months after GKRS (range 8.7-11.4 months), and they concluded that GKRS may be a good treatment option for the local control of metastatic lesions and for improved survival time in patients with multiple metastatic brain lesions, even in those patients harboring more than 15 metastatic brain lesions.
In this study, we analyzed the usefulness of multiple GKRS by studying on the survival time between patients with multiple GKRS (group A) and patients with a single GKRS plus whole brain radiation therapy (WBRT) (group B). And we also investigated four factors in patients with multiple GKRS to find the factors associated with survival time in group A : 1) the number of lesions in each patient, 2) the average volume of lesions in each patient, 3) the number of repeated GKRS, and 4) the interval of development of new lesions, respectively.
Between May 2006 and July 2010, 112 patients with brain metastases from lung cancer underwent GKRS. Metastatic brain lesions were identified with 3.0 Tesla MRI (Verio, Siemens, Erlangen, Germany). This study included 31 patients who had multiple metachronous metastases. Twenty out of 31 patients underwent multiple GKRS (group A) and the remaining 11 patients underwent a single GKRS plus WBRT (group B). Inclusion criteria were as follows : 1) only intracranial metastases in additional positron emission tomography at the time of their first GKRS treatment to eliminate the effect of systemic condition, 2) the Karnofsky Performance Scale (KPS) at each GKRS scored 70 or more, 3) patients who did not underwent WBRT prior to GKRS. Patients in group B were the patients who did not want repeated GKRS due to economic problem and they showed 70 or more score of KPS and only intracranial metastases at time of WBRT. The patients continued receiving chemotherapy until they or their family refused further treatment. Chemotherapy regimens were different according to histopathologic types of the lung cancer.
The Leksell Gamma Knife, C-type (Elekta Instruments, Inc.) was used for all patients' treatments. We obtained T1-weighted, high-dose gadolinium (Gd) enhanced images, with an injected Gd volume of 0.4 mmol/kg. Furthermore, we used multiple isocenters to insure a highly conformal dose distribution and defined a prescription dose as a dose covering at least 93% of the target volume. To irradiate the tumor margins, we used the 50% or 48% isodose line, with an average marginal dose of 20 Gy (range 14-22). WBRT was performed by radiation oncologist. Irradiated dosage was total 20 Gy for 10 days (200 cGy per day).
The follow-up schedule was the same in all patients. Patients underwent brain MRI with a 3.0 Tesla MRI (Verio, Siemens, Erlangen, Germany) every 3 months after GKRS treatment and received clinical and neurological examinations every month. In patients with severe headache or neurological deterioration, we obtained brain MRI every month.
We analyzed cumulative survival between two groups of multiple GKRS (group A) and a single GKRS plus WBRT (group B) by Kaplan-Meier method. In group A, we used the Kaplan-Meier and Cox proportional hazards method to analyze relationship between survival and 1) the number of lesions in each patient, 2) the average volume of lesions in each patient, 3) the number of repeated GKRS, and 4) the interval of development of new lesions, respectively. Total volume of lesions of individual patients was calculated. However, it was not statistically analyzed because of the diversity in volumetric measurement in each patient and each lesion.
In group A, four factors were analyzed as followed standards : 1) the number of lesions in each patient : above 10 and below 10, 2) the average volume of lesions in each patient : above 10 cc and below 10 cc, 3) number of repeated GKRS : above 3 times and below 3 times, and 4) the interval of development of new lesions : above 7 months and below 7 months.
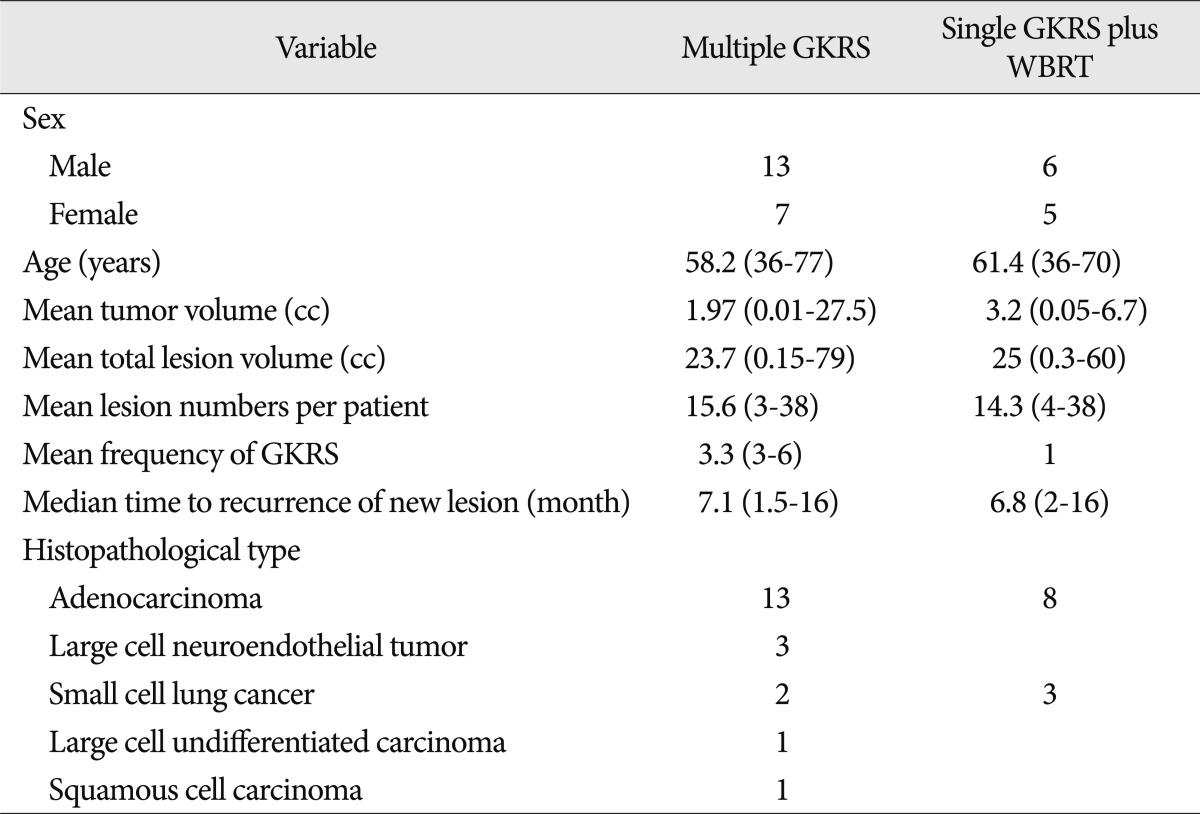
The patients' male to female ratio was 13 : 7, and their mean age was 58.2 years (range 36-77 years). Mean tumor volume was 1.97 cc (range 0.01-27.5 cc) and mean lesion number per patients was 15.6 lesions (range 3-38 lesions). Total treated lesion volume was average 23.7 cc (range 0.15-79 cc). Average frequency of GKRS treatments was 3.3 times (range 3-6 times). Median time for development of new lesions was 7.1 months (range 1.5-16 months). Histopathological findings regarding lung cancers revealed adenocarcinoma in 13 patients, large cell neuroendocrine tumor in three, small cell lung cancer in two, large cell undifferentiated carcinoma in one, and squamous cell cancer in one (Table 1). Median survival time after first GKRS was 18 months (range 6-50 months). Three patients had been survived for quite a long time : 47, 48, and 50 months after their first GKRS, respectively. Patients did not suffer from any radiation-induced adverse effects. From the neurological perspective, two patients with large, centrally-located lesions showed temporary motor weakness after GKRS. Causes of death were medical problems that included aggravation of the primary lung cancer, pneumonia, and cachexia. Neurological deterioration was not a cause of patient death. The mean follow-up duration was 18 months (range 6-60 months).
Male to female ratio was 6 : 5, and their mean age was 61.4 years (range 36-70 years). Mean tumor volume was 3.2 cc (range 0.05-6.7 cc) and mean lesion number per patient was 14.3 lesions (range 4-38 lesions). Total treated lesion volume was average 25 cc (range 0.3-60 cc). Median time for development of new lesions was 6.8 months (range 2-16 months). Histopathological findings regarding lung cancer revealed adenocarcinoma in 8 patients, small cell lung cancer in 3 (Table 1). Median survival time after first GKRS was 6 months (range 3-18 months).
Causes of death were medical problems in 8 patients that included aggravation of the primary lung cancer, pneumonia, and efficacachexia. Neurological deterioration was the cause of death in 3 patients. The mean follow-up duration was 8 months (range 3-18 months).
The cumulative survival graph with Kaplan-Meier method of patients with single GKRS plus WBRT showed a stiff-declining curve compared with the curve of multiple GKRS (Fig. 1) and p-value was lesser than 0.05.
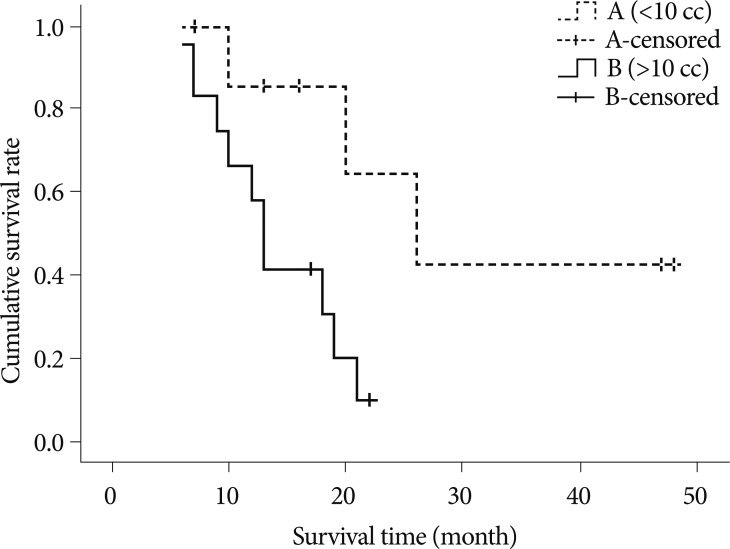
Concerning the number of the lesions (above 10 and below 10) in patients with multiple GKRS, p-value is 0.7595. In terms of the average volume of the lesions (above 10 cc and below 10 cc), the p-value is 0.0243 (Fig. 2). Survival curve of patients with the average volume of lesions above 10 cc showed the stiff-declining curve compared with the curve of below 10 cc. In viewing the repeated number of GKRS (more than 3 times and less than 3 times), the p-value is 0.0885. In pointing out the interval of development of new lesions (more than 7 months and less than 7 months), the p-value is 0.1769.
All variables are significant because the p-value of them is less than 0.05 under the 95% significant level according to the regression coefficient (Table 2). According to these results, authors realized that the proportional hazard of the total number of lesion is 1.1559. In the case of the average volume of lesions, the proportional hazard is shown 1.0005. The proportional hazard of the repeated number of GKRS is 0.08943. We found that the proportional hazard for the interval of development of new lesions is 0.5970.
Many recent study series on patients with various pathologies report survival times of 7-10.5 months for single or multiple newly-diagnosed or recurrent brain metastases9,12,15). Such patients with brain metastases from lung cancer have median survival time of 10-12 months3,8,18). Aoyama et al.1) reported patients with 1-4 brain metastases undergoing stereotactic radiosurgery (SRS) treatment alone had median survival times of 8 months, while patients receiving SRS plus WBRT survived 7.5 months. This result confirms the efficacy of SRS alone, even for patients with 1-4 brain metastases1,2). Hoffman's group reported 10 month survival for patients with recurrent brain metastases after SRS10). Karlsson et al.13) presented no statistically significant relationship between number of brain metastases upon first treatment and total number of GKRS treatments. They found long-term survivors among patients with 4 or more cerebral metastases who underwent GKRS. Among patients with 4 or more metastases, 21% of patients survived for 1 year after treatment, 10% survived for 27 months, and 5% survived for 43 months. Bhatnagar et al.5) reported the median overall survival time after SRS for all patients having 4 or more intracranial metastases was 8 months. They concluded radiosurgery seems to provide a survival benefit for patients having 4 or more lesions, and total treatment volume is the most significant survival predictor. Chang et al.6) analyzed GKRS's effectiveness for multiple metastatic brain tumors. They presented a median patient survival time of 10 months (range of 8.7-11.4 months), and they concluded GKRS might be a good treatment option for local metastatic lesion control and for improving survival time in patients with multiple metastatic brain lesions, even in those patients harboring more than 15 metastatic brain lesions.
Our patients with multiple GKRS showed an average of 15.6 lesions (range 3-38 lesions) each and received an average of 3.3 times (range 3-6 times) of GKRS. Median survival time of patients with multiple GKRS after their first GKRS was 18 months (range 6-50 months). It is almost three folds of survival time rather than that of patients with a single GKRS plus WBRT. This result supports that multiple GKRS treatments for metachronous multiple lesions are of necessity for treatment strategy for patients with metastatic brain tumors with regard to extending survival time and avoiding delayed adverse effects after WBRT. Furthermore, maintenance of good general condition of patients was of importance to endure chemotherapy and other advanced treatments, and it provided the opportunities of extension of survival.
Three class I studies demonstrate that WBRT significantly lowers the risk of a distant recurrence comparing to local tumor therapies (SRS or surgical resection) used in isolation1,14,16). However, four of 10 retrospective cohort studies revealed no significant differences in distant recurrence rates between these two treatment strategies7,11,17,19). Our results revealed that median time to recurrence was 7.1 months (range 1.5-16 months, the average frequency of GKRS treatments per patient was 3.3 times) in patients with multiple GKRS and 6.8 months (range 2-16 months) in patients with a single GKRS plus WBRT. These data implicated that GKRS made a longer average progression-free time, in comparison to the other reports on progression-free time of WBRT which was 4.6-6 months7). This result supports that GKRS alone may be an effective modality for controlling metastatic brain tumors as much as upfront WBRT.
Cox proportional hazard analysis showed that the proportional hazard of the number of the lesions, the average volume of lesions in each patients, the repeated number of GKRS, and the interval of development of new lesions were 1.1559, 1.0005, 0.08943, and 0.5970, in each. From these results we can implicate that patients with the every additional number of the lesions are more risky in 1.1559 times in the number of the lesions, when other variables are controlled. In the aspect of the average volume of lesions in each patient, the proportional hazard can be understood that patients with every additional unit volume (additional 1 cc) are more risky in 1.0005 times. With respect to the repeated number of GKRS, the proportional hazard of 0.08943 means that patients with the lesser number of GKRS are more risky. The proportional hazard (0.5970) for the interval of development of new lesions between the times of each GKRS means that the shorter the recurrence duration is, the higher the risk is in patients.
Even though our study had some limitations in the small number of patients and the heterogeneous histopathologic findings of lung cancer, this result showed that multiple GKRS is valuable treatment in extending the survival time in patients with multiple metachronous brain metastases of lung cancer.
Our study reports that patients with multiple GKRS showed 18 months of median survival time after first GKRS and, on the contrary, patients with a single GKRS plus WBRT showed 6 months of median survival time after first GKRS. In patients with multiple GKRS, the result of Kaplan-Meier analysis showed that the large numbers of lesion (more than 10 lesions), the large repeated numbers of GKRS (more than three times) and even the short interval of development of new lesions (shorter than 7 months) did not influence onto the limitation of the survival time of patients. The large volume of the lesions was only related with shortening the survival time of patients. In Cox proportional hazard method, however, the volume of the lesions was not a hazard factor of shortening the survival time. Authors thought that multiple GKRS could improve or sustain a general and neurological condition of patients without radiation induced adverse effect and it made opportunities that patients get advanced chemotherapy and other supportive treatments. In conclusion, our result supports that multiple GKRS is of value of treatment in extending the survival time in patients with multiple metachronous brain metastases of lung cancer with good performance status, and that the number of the lesions and the frequency of development of new lesions are not an obstacle in treating patients with GKRS.
References
1. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases : a randomized controlled trial. JAMA. 2006; 295:2483–2491. PMID: 16757720.

2. Aoyama H, Tago M, Kato N, Toyoda T, Kenjyo M, Hirota S, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007; 68:1388–1395. PMID: 17674975.

3. Auchter RM, Lamond JP, Alexander E, Buatti JM, Chappell R, Friedman WA, et al. A multiinstitutional outcome and prognostic factor analysis of radiosurgery for resectable single brain metastasis. Int J Radiat Oncol Biol Phys. 1996; 35:27–35. PMID: 8641923.

4. Bahl A, Kumar M, Sharma DN, Jothy Basu KS, Jaura MS, Rath GK, et al. Reirradiation for progressive brain metastases. J Cancer Res Ther. 2009; 5:161–164. PMID: 19841556.

5. Bhatnagar AK, Flickinger JC, Kondziolka D, Lunsford LD. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006; 64:898–903. PMID: 16338097.

6. Chang WS, Kim HY, Chang JW, Park YG, Chang JH. Analysis of radiosurgical results in patients with brain metastases according to the number of brain lesions : is stereotactic radiosurgery effective for multiple brain metastases? J Neurosurg. 2010; 113:73–78. PMID: 21121789.

7. Combs SE, Schulz-Ertner D, Thilmann C, Edler L, Debus J. Treatment of cerebral metastases from breast cancer with stereotactic radiosurgery. Strahlenther Onkol. 2004; 180:590–596. PMID: 15378190.

8. Flickinger JC, Kondziolka D, Lunsford LD, Coffey RJ, Goodman ML, Shaw EG, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. 1994; 28:797–802. PMID: 8138431.

9. Flickinger JC, Lunsford LD, Somaza S, Kondziolka D. Radiosurgery : its role in brain metastasis management. Neurosurg Clin N Am. 1996; 7:497–504. PMID: 8823777.
10. Hoffman R, Sneed PK, McDermott MW, Chang S, Lamborn KR, Park E, et al. Radiosurgery for brain metastases from primary lung carcinoma. Cancer J. 2001; 7:121–131. PMID: 11324765.
11. Jawahar A, Willis BK, Smith DR, Ampil F, Datta R, Nanda A. Gamma knife radiosurgery for brain metastases : do patients benefit from adjuvant external-beam radiotherapy? An 18-month comparative analysis. Stereotact Funct Neurosurg. 2002; 79:262–271. PMID: 12890985.

12. Joseph J, Adler JR, Cox RS, Hancock SL. Linear accelerator-based stereotaxic radiosurgery for brain metastases : the influence of number of lesions on survival. J Clin Oncol. 1996; 14:1085–1092. PMID: 8648361.

13. Karlsson B, Hanssens P, Wolff R, Söderman M, Lindquist C, Beute G. Thirty years’ experience with Gamma Knife surgery for metastases to the brain. J Neurosurg. 2009; 111:449–457. PMID: 19199505.

14. Muacevic A, Wowra B, Siefert A, Tonn JC, Steiger HJ, Kreth FW. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain : a randomized controlled multicentre phase III trial. J Neurooncol. 2008; 87:299–307. PMID: 18157648.

15. Noël G, Proudhom MA, Valery CA, Cornu P, Boisserie G, Hasboun D, et al. Radiosurgery for re-irradiation of brain metastasis : results in 54 patients. Radiother Oncol. 2001; 60:61–67. PMID: 11410305.

16. Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, et al. Postoperative radiotherapy in the treatment of single metastases to the brain : a randomized trial. JAMA. 1998; 280:1485–1489. PMID: 9809728.
17. Sneed PK, Lamborn KR, Forstner JM, McDermott MW, Chang S, Park E, et al. Radiosurgery for brain metastases : is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999; 43:549–558. PMID: 10078636.

18. Sneed PK, Suh JH, Goetsch SJ, Sanghavi SN, Chappell R, Buatti JM, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002; 53:519–526. PMID: 12062592.

19. Varlotto JM, Flickinger JC, Niranjan A, Bhatnagar A, Kondziolka D, Lunsford LD. The impact of whole-brain radiation therapy on the long-term control and morbidity of patients surviving more than one year after gamma knife radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2005; 62:1125–1132. PMID: 15990018.

20. Wong WW, Schild SE, Sawyer TE, Shaw EG. Analysis of outcome in patients reirradiated for brain metastases. Int J Radiat Oncol Biol Phys. 1996; 34:585–590. PMID: 8621282.

Fig. 1
Cumulative Kaplan-Meier survival curves for patients with multiple GKRS (dashed line) and patients with single GKRS plus WBRT (solid line) after first gamma knife radiosurgery. Cumulative survival curve of patients with single GKRS plus WBRT showing stiff-declining curve compared with curve of multiple GKRS. GKRS : gamma knife radiosurgery, WBRT : whole brain radiation therapy.
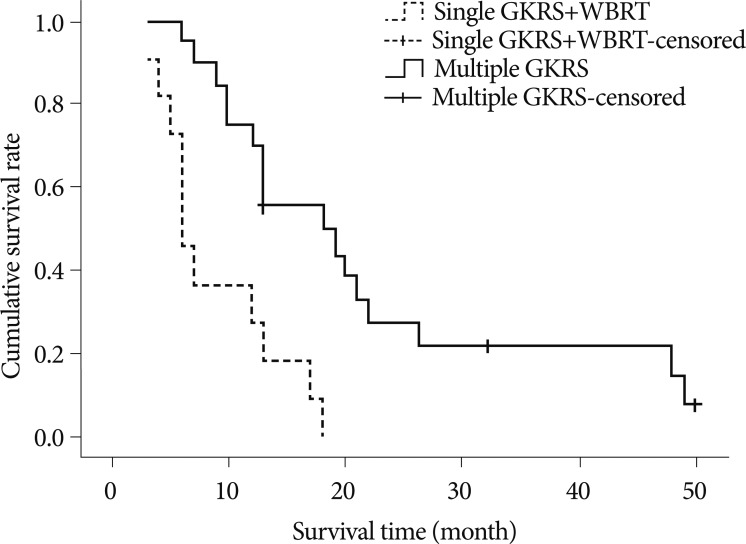
Fig. 2
Kaplan-Meier survival curves for patients with multiple GKRS according to the average volume of lesions (above 10 cc, solid line and below 10 cc, dashed line). Survival curve of patients with the average volume of lesions above 10cc showing stiff-declining curve compared with curve of below 10 cc. GKRS : gamma knife radiosurgery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download