Abstract
Objective
In patients with spontaneous intracerebral hemorrhage (ICH), the risk factors for seizure and the effect of prophylactic anticonvulsants are not well known. This study aimed to determine the risk factor for seizures and the role for prophylactic anticonvulsants after spontaneous ICH.
Methods
Between 2005 and 2010, 263 consecutive patients with spontaneous ICH were retrospectively assessed with a mean follow-up of 19.5 months using medical records, updated clinical information and, when necessary, direct patient contact. The seizures were classified as early (within 1 week of ICH) or late (more than 1 week after ICH). The outcomes were measured with the Glasgow Outcome Scale at discharge and the modified Rankin Scale (mRS) at both 2 weeks and discharge.
Results
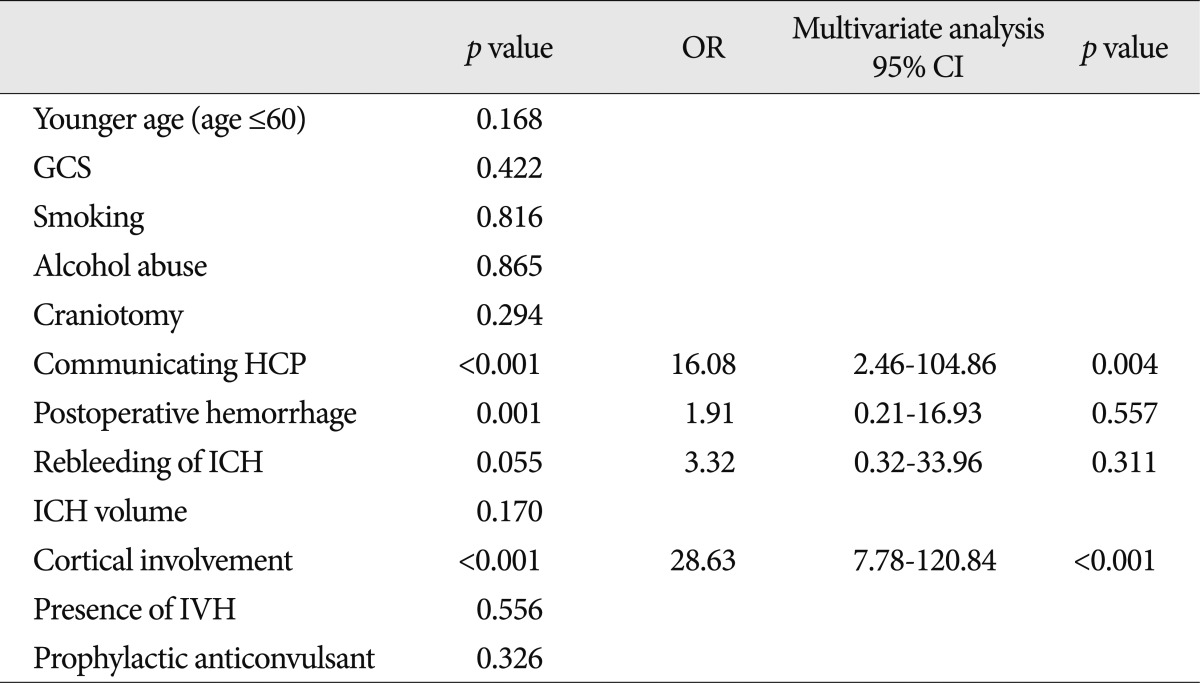
Twenty-two patients (8.4%; 9 patients with early seizures and 13 patients with late seizures) developed seizures after spontaneous ICH. Out of 263 patients, prophylactic anticonvulsants were administered in 216 patients. The prophylactic anticonvulsants were not associated with a reduced risk of early (p=0.094) or late seizures (p=0.326). Instead, the factors associated with early seizure were cortical involvement (p<0.001) and younger age (60 years or less) (p=0.046). The risk of late seizure was increased by cortical involvement (p<0.001) and communicating hydrocephalus (p=0.004). The prophylactic anticonvulsants were associated with a worse mRS at 2 weeks (p=0.024) and at last follow-up (p=0.034).
The incidence of seizures following spontaneous intracerebral hemorrhage (ICH) reportedly ranges from 2.8-18.7%2,4-6,8,12,14,19,28). Precise estimates of the risk of developing seizures would be helpful not only to patients but also to those advising the patients on their return to work. Several studies have reported that the factors for provoked seizures following spontaneous ICH are related to ICH volume, frontal location, and cortical involvement8,33). Several studies have reported on risk factors for provoked seizures following spontaneous ICH, but these studies have included relatively few patients8,14).
Because not all patients experience seizures after spontaneous ICH, there has been a continuous debate regarding the effects of prophylactic anticonvulsants. Recent studies have reported that the administration of prophylactic anticonvulsants in ICH patients has no significant effect on the risk of early seizures. However, some studies have suggested that the administration of prophylactic anticonvulsants in lobar ICH patients decreases the risk of early seizures7,23,26).
The present study was conducted to investigate the risk factors for seizures in spontaneous ICH patients and the effects of prophylactic anticonvulsants on seizures and the patient's functional outcome.
Patients who were newly diagnosed with a spontaneous ICH at our hospital from the year 2005 to 2010 were included and analyzed retrospectively. The ICH diagnosis was based on computed tomography (CT) scans or magnetic resonance imaging. The patient data were prospectively recorded in the ICH database at the time of diagnosis and at each follow-up visit. The ICH database at our hospital is a prospective enrollment database that contains demographic, clinical, radiographic, and treatment data on all patients diagnosed with ICH in our hospital since 2005. All records regarding subsequent hospitalization and death were reviewed, and the relevant events occurring during follow-up visits, including changes in anticonvulsant therapy, were also recorded. Patients with histories of seizure, stroke, infection of the central nervous system, traumatic hemorrhage, metabolic disorders, brain tumor, hemorrhage due to arteriovenous malformation, subarachnoid hemorrhage, cerebellar hemorrhage or brain stem hemorrhage were excluded (history of seizure in 3 patients, history of stroke in 9 patients, metabolic disorders in 2 patients, brain tumor in 5 patients, arteriovenous malformation in 3 patients, traumatic hemorrhage in 9 patients, subarachnoid hemorrhage in 7 patients, and cerebellar hemorrhage or brain stem hemorrhage in 6 patients). A total of 307 patients were enrolled in our ICH database from the year 2005 to 2010. Of these 307 patients, 263 remained eligible for analysis after the exclusion criteria were applied. The baseline characteristics are shown in Table 1.
The Institutional Review Board of our hospital approved all contents of the study. Informed consent was not required from these patients because the study depended only on information obtained as part of their routine clinical care and patient medical records.
The seizures were classified according to the recommendations of the International League Against Epilepsy, and seizures were classified as simple partial, complex partial, and partial with secondary generalization (or generalized) seizures25). Status epilepticus was defined as continuous behavioral seizure activity or repetitive seizures without full recovery of neurological function between seizures occurring over a period longer than 30 minutes29). The diagnosis was based on direct observation of the seizure by a physician at the time of hospitalization or the history of the neurologist in charge of the patient according to reliable descriptions obtained from either ambulance personnel when the seizure occurred during transportation or the patient or his/her close family members when the seizure occurred at home. An electroencephalogram was not required for inclusion in the study but was performed if possible after admission. If clinically symptomatic events (sudden onset of headache, seizure, focal deficits, or a combination of these events) occurred, a CT scan was generally performed to determine the cause of events, such as rebleeding. For the purpose of this study, the following clinical events were considered : craniotomy for ICH evacuation, CT scan-confirmed rebleeding, and hydrocephalus. Seizures occurring soon after clinical events were classified as being associated with these events.
We classified seizures according to the onset of clinical ictus. Early seizures were defined according to the ILAE guidelines as any seizure occurring within 1 week of spontaneous ICH onset25), and late seizures were defined as occurring more than 1 week later. In addition, among early seizures, seizures occurring within 6 hours of ICH onset were defined as immediate seizures.
The administration of prophylactic anticonvulsants to patients who had not had a seizure was based on the physician's medical attitude towards seizure prophylaxis. Some physicians discontinued prescription of anticonvulsants after 2-week period of medication while others prescribed anticonvulsants only in patients who presented seizures. The prophylactic anticonvulsants included valproate, levetiracetam, and phenytoin. In general, prophylactic anticonvulsants were administered immediately at the time of ICH diagnosis and discontinued after 2 weeks10,20,22). When a clinical seizure occurred after the discontinuation of anticonvulsants, the same medications were resumed. In patients who were not initially prescribed with prophylactic anticonvulsants, anticonvulsants were given to patients who presented with acute symptomatic seizures, and were discontinued gradually over 3-month period in patients with no seizure for 1 year.
CT scans were performed at admission for all patients. ICH was classified as deep ICH (basal ganglia, internal capsule, and thalamus), deep ICH with intraventricular hemorrhage (IVH), deep ICH with lobar extension, deep ICH with lobar extension and IVH, and lobar ICH (frontal, temporal, parietal, and occipital) (Fig. 1-3). The cortical involvement of ICH was defined as any ICH that extended to the cerebral cortex (Fig. 4). The images were reviewed by a single neuro-radiologist who was blinded to the clinical data and was not involved in patient management. We measured ICH volume using the admission CT scan according to the ABC/2 method18).
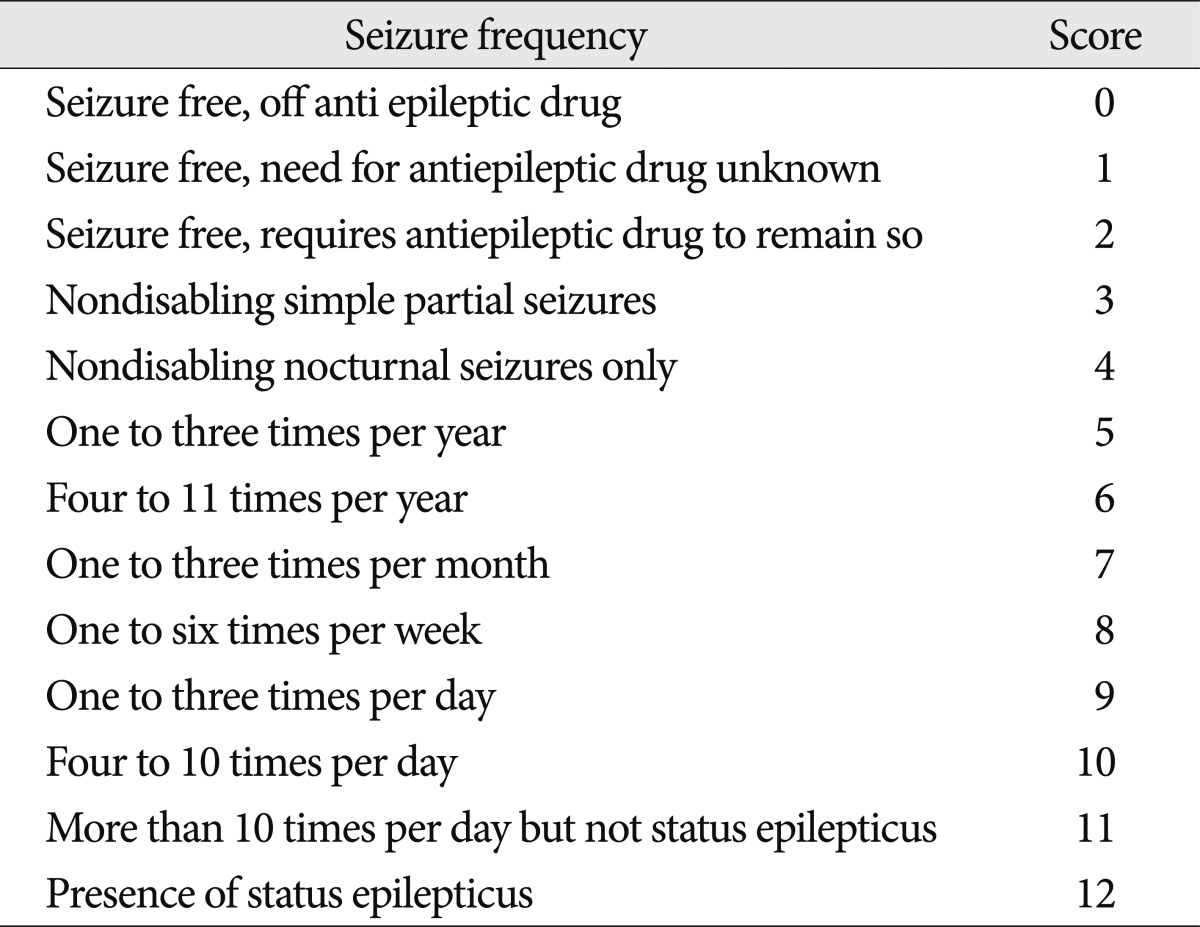
The outcomes were measured with the Glasgow Outcome Scale (GOS) at discharge and the modified Rankin Scale (mRS) at both 2 weeks after the onset of ICH and discharge3,15). The mRS was used in the outpatient clinic or during a telephone interview with the physician or caregiver in charge of the patient. We assessed seizure outcome using the Engel seizure frequency scoring system at the last follow-up (Table 2)30).
The associations between categorical variables and seizure occurrence during the study period were analyzed using the Pearson χ2 test, and Fisher's exact test when the number of patients in a group was 5 or less (i.e., number of the patients with communicating hydrocephalus). Continuous variables were compared using Student's t-test. The prognostic factors considered for univariate analysis included patient demographics, details of ICH, craniotomy for ICH evacuation, CT scan-confirmed rebleeding, obstructive hydrocephalus, communicating hydrocephalus, and cerebral edema. Multivariable logistic regression analysis was used to assess the association of variables with seizure occurrence. The differences in functional outcome were compared between groups using Student's t test and the Mann-Whitney test. The results are presented as the adjusted odds ratio (OR) with a 95% confidence interval (CI). The level of significance was set as p<0.05.
The median follow-up period was 19.5 months (range, 0.1-76.3 months). Detailed descriptions of the ICHs in the 263 patients are provided in Table 1. Treatment options for spontaneous ICH included craniotomy for ICH evacuation and external ventricular drainage (EVD)/ventriculoperitoneal shunt (VPS) for hydrocephalus. Sixty-nine patients underwent a craniotomy, an EVD was placed in 32 patients, and 9 patients underwent VPS. Five patients underwent craniotomy and EVD, 3 patients EVD and shunt, and 1 patient craniotomy, EVD and shunt. Of the 100 patients who underwent surgical treatment, 12 experienced postoperative hemorrhages.
Of 263 patients, prophylactic anticonvulsants were administered in 216 patients (82.1%). There were no significant differences between patients who received prophylactic anticonvulsants and those patients who did not (Table 1).
Seizures occurred in 8.4% (22/263) of patients, including early seizures in 9 patients and late seizures in 13 patients. The interval from the onset of spontaneous ICH to seizure was 0-810 days (mean, 126 days). Of the 9 patients with early seizures, 3 patients presented immediate seizures. Among all patients who experienced seizures, the type of seizures presented were simple partial seizures in 8 patients, complex partial seizures in 3 patients, partial with secondary generalization or generalized seizures in 10 patients, and status epilepticus in 1 patient. The clinical events preceding the seizures were observed in 6 patients and included surgical evacuation of the hematoma in 2 patients, rebleeding in 2, and communicating hydrocephalus in 2.
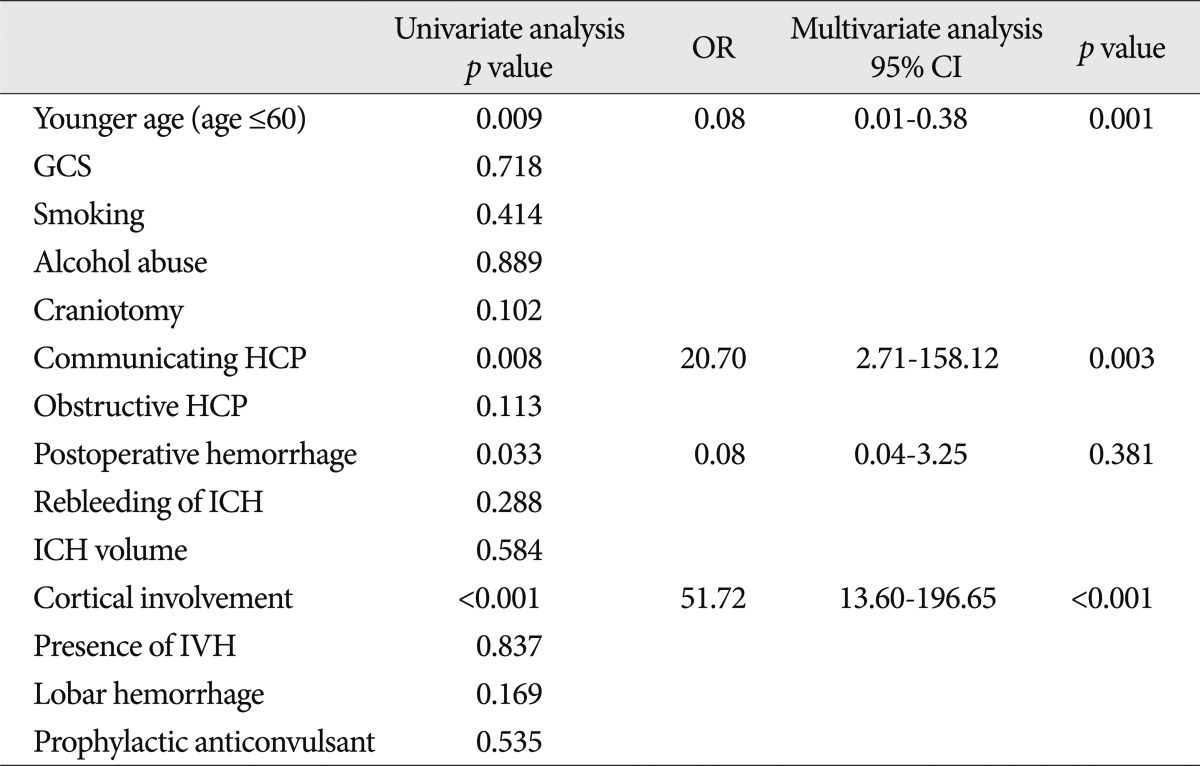
When the two groups with (n=22) and without (n=241) seizures were compared, the patients with seizures were significantly younger age (60 years or less) and were significantly more likely to have postoperative hemorrhage, cortical involvement, and communicating hydrocephalus than patients without a seizure (Table 3). After multivariate analysis, cortical involvement (OR=51.72; 95% CI 13.60-196.65), younger age (60 years or less) (OR=0.08; 95% CI 0.01-0.38), and communicating hydrocephalus (OR=20.70; 95% CI 2.71-158.12) appeared to be independent clinical factors for developing epileptic seizures.
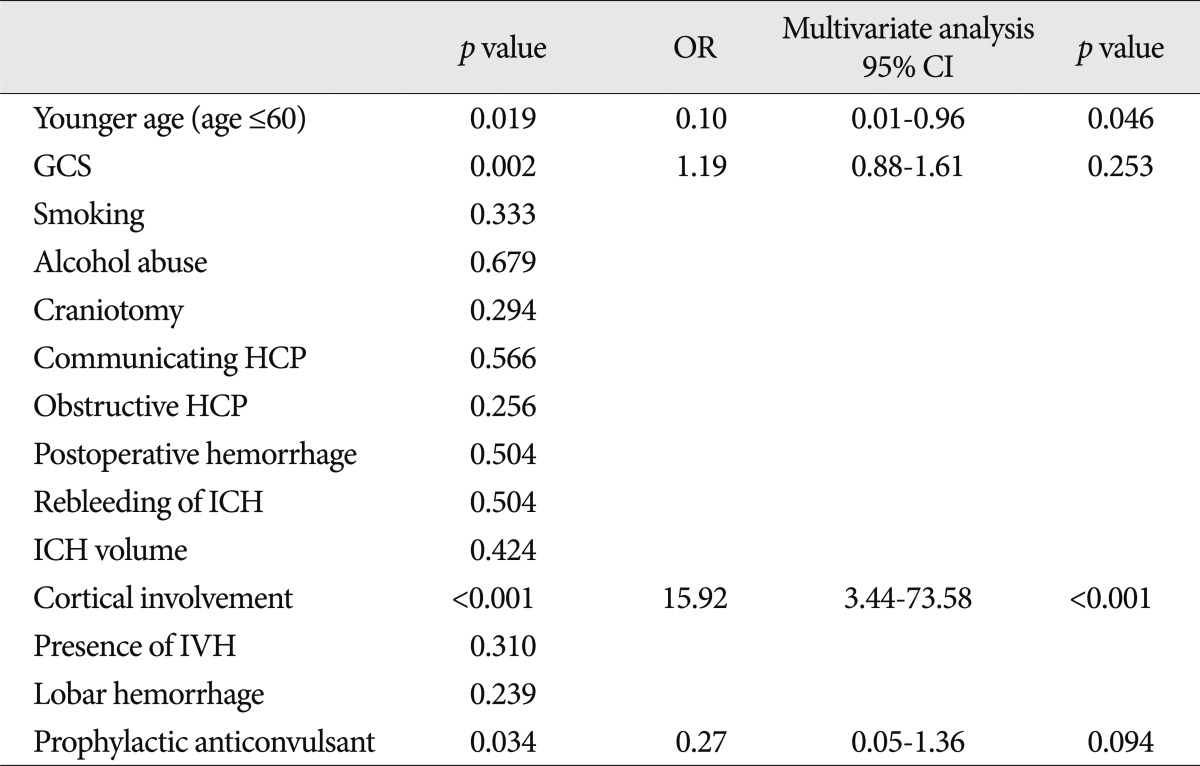
The factors associated with early seizure are presented in Table 4. In a multivariate analysis, the factors independently associated with the occurrence of early seizures were cortical involvement (OR=15.92; 95% CI 3.44-73.58) and younger age (60 years or less) (OR=0.10; 95% CI 0.01-0.96).
The factors associated with late seizure are presented in Table 5. In the multivariate analysis, the factors independently associated with the occurrence of late seizures were cortical involvement (OR=28.63; 95% CI 7.78-120.84) and communicating hydrocephalus (OR=16.08; 95% CI 2.46-104.86).
At day 7 after the ICH, 20 of the 263 patients had died. Nineteen patients died from hemorrhage, and 1 patient died after acute myocardial infarction. At the last follow-up, 34 patients had died. A total of 28 patients died from hemorrhage, 2 patients died from renal failure, 2 patients died from acute myocardial infarction, 1 patient died from acute respiratory distress syndrome, and 1 patient died from sepsis. The occurrence of seizure did not influence mortality at 7 days (p=0.564) or at the last follow-up (p=0.732).
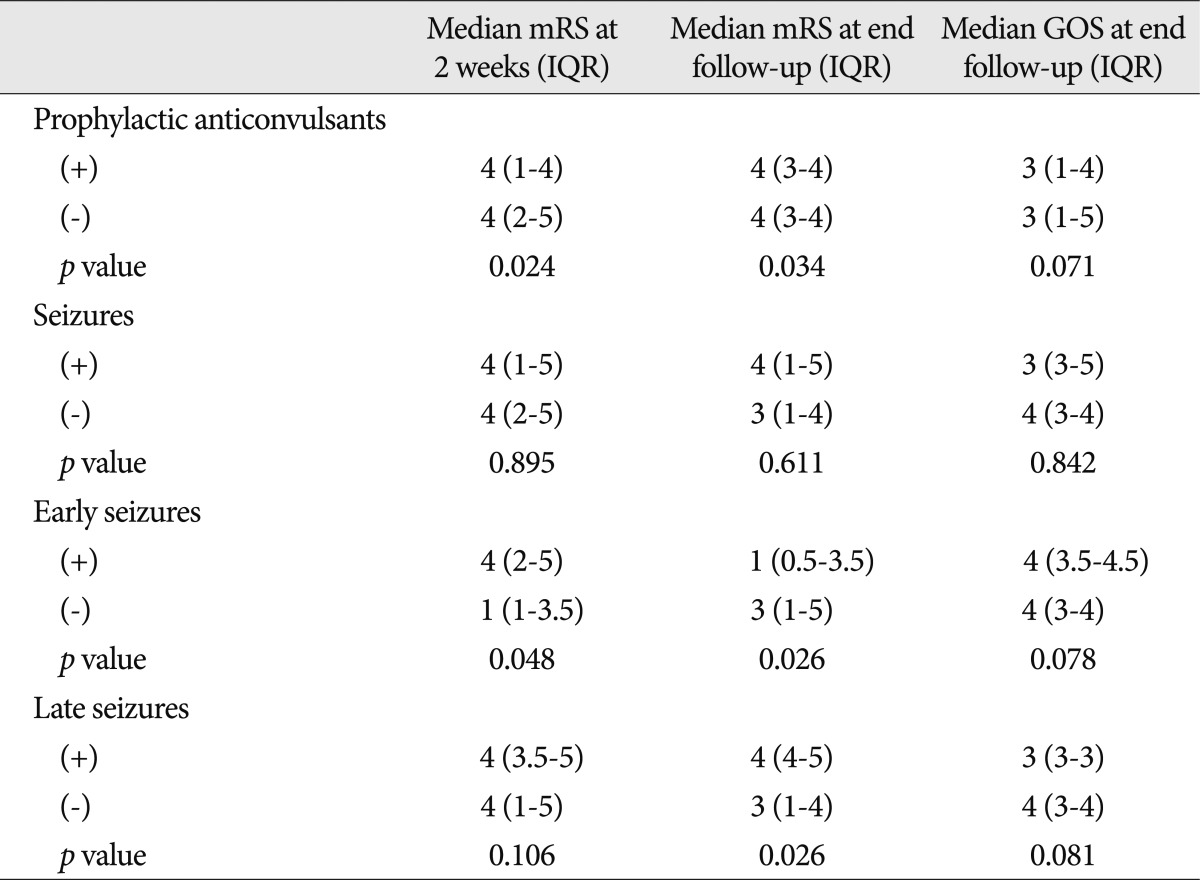
The administration of prophylactic anticonvulsants had a tendency to decrease the incidence of early seizures (p=0.094) but was not associated with the prevention of provoked seizures (p>0.1 for all) (Table 3, 4, 5). The administration of prophylactic anticonvulsant was associated with a worse mRS at 2 weeks after the onset of ICH (p=0.024) and at the last follow-up (p=0.034) but was not associated with a worse GOS at the last follow-up (p=0.071) (Table 6).
Of the 22 patients who developed seizures, 1 patient with a late seizure died from sepsis 13 days after the onset of ICH. At the last follow-up evaluation of the remaining 21 patients, 90.5% (19/21) of patients were seizure-free. Of the 9 patients with early seizures, 7 achieved the Engel seizure frequency score of 0, and 2 achieved a score of 2. Of the patients with late seizure, 10 patients were medication-free (score 0), and 2 patients had a score of 3.
Seizures were not associated with functional outcomes (p>0.1 for all) (Table 6). However, the mRS at 2 weeks after the onset of ICH and the GOS at the last follow-up in patients with early seizure were worse than patients without early seizure (p<0.05 for all), but the difference of mRS at the last follow-up between the 2 groups failed to reach significance (p=0.078). When comparing the outcomes between patients with and without a late seizure, a significant difference was not found in mRS at 2 weeks after the onset of ICH (p=0.106) or in GOS at the last follow-up (p=0.081), but was found in mRS at the last follow-up (p=0.026).
Among the 182 patients prescribed with valproic acid, serum ammonia level was evaluated in 20 patients who presented deterioration of consciousness without specific causes, and hyperammonemia (ammonia >80 µg/dL) was identified in 8 patients. Of the 30 patients prescribed with levetiracetam, thrombocytopenia (platelet <140×103/µL) was revealed in 4 patients among whom 1 patient undwent craniotomy due to increased amound of intracerebral hemorrhage. From the 4 patients prescribed with phenytoin, 1 patient presented fever with no definite infection focus. However, no statistical significance was identified concerning correlation of mRS and GOS at last follow-up examination between patients with and without complications among the group in which prophylactic anticonvulsants were prescribed.
We found that, although 8.4% (22/263) of patients with spontaneous ICH experienced seizures during the follow-up period, epilepsy was a sequela in only 0.8%, except for one patient who died. Early seizures occurred in 3.4% of patients, and late seizures occurred in 5.0% of patients. Previous studies have reported the incidence rate of seizures occurring after spontaneous ICH to be 2.8-18.7%2,4-6,8,14,19,28). When comparing the incidence of seizures reported in previous literatures, our study revealed consistent results.
Several previous studies examined early seizures and late seizures occurring after stroke10,28,31). In this current study, younger age and cortical involvement were independent factors for early seizures. Post-hemorrhagic communicating hydrocephalus and cortical involvement were independent factors for late seizures. Previous studies have reported that the cortical involvement of ICH affects the incidence of seizures5,7,8,11,17,31,33). The pathogenesis of seizures in cortical involvement may involve the location of the lesion in the gray-white matter interface, creating a condition similar to the surgical isolation of cortex, which results in its sustained paroxysmal activity9), and direct irritation of the cortex is one possible mechanism through which post-ICH seizures are provoked32).
In the present study, young age (60 years or less) and communicating hydrocephalus were risk factors for seizures after spontaneous ICH. But the exact cause was unclear. Some authors suggested younger age is one of the risk factors for seizures in ICH, however, the pathogenesis was not noted in the literature7,33). Communicating hydrocephalus was also suggested as one of the risk factors for seizures in ICH16,24). In common with age, the exact cause was also still unclear.
The effect of ICH volume on the occurrence of seizures after spontaneous ICH remains controversial. In a previous study, Yang et al.33) stated that large ICH volumes were significantly correlated with the occurrence of early seizures. In a prospective study by De Herdt et al.7), ICH volume was unrelated to the occurrence of seizures. Several pathophysiological mechanisms, although requiring further elucidation, have been implicated in the occurrence of seizures after spontaneous ICH, including a combination of the sudden development of a space-occupying lesion with mass effect, focal ischemia, and blood products, which could explain the occurrence of seizures in the early phase of hemorrhagic stroke5). However, in this study, large ICH volume was not associated with the occurrence of seizures.
The effect of seizures on the outcomes of patients with spontaneous ICH is controversial. Arboix et al.1) reported poor outcomes in cases of seizures following stroke. In other studies, no significant influences on outcomes were evident23). In this study, patients with early seizures had worse functional outcomes at both 2 weeks after the onset of ICH and the last follow-up than those patients without seizures. Regarding late seizures, there was no difference between the 2 groups at 2 weeks after the onset of ICH, but on last follow-up, poor outcomes were apparent in patients with late seizures. Although seizures did not influence the mortality, it could lead to poor functional outcome. Passero et al.23) reported that the administration of prophylactic anticonvulsants decreases the risk of acute seizures, but Reddig et al.26) stated that prophylactic anticonvulsants have no significant benefits. In this study, the incidence of early seizures tended to decrease in patients prescribed prophylactic anticonvulsants. However, the administration of prophylactic anticonvulsants did not prevent the occurrence of seizures, and the outcomes were rather poor in patients prescribed prophylactic anticonvulsants. The reasons or risk factors for poor functional outcomes in patients prescribed with prophylactic anticonvulsants were not identified in this study. However, adverse factors including hyperammonemia and thrombocytopenia were present in patients prescribed with prophylactic anticonvulsants. Upon review of previous studies, complications including hyperammonemia and thrombocytopenia could be caused by valproate or levetiracetam13,27). One study demonstrated an association between phenytoin prophylaxis and more fever, worse clinical examination, and a worse functional outcome at follow-up21). Based on our findings and the findings of others, the benefit of prophylactic anticonvulsant therapy would further be limited because of the relatively easy control of seizures if they did occur and the adverse effects of anticonvulsant therapy. Therefore, to balance the limited benefit and the potential serious adverse effects of antiepileptic drugs, prophylactic anticonvulsant must be used with caution only when necessary; future research may clarify protocols for the effective use of anticonvulsants and target specific populations at high risk for seizures after ICH (e.g., ICH with cortical involvement and depressed mental status).
Our study has some limitations that should be considered. First, we performed a retrospective observational study. Second, because the follow-up period was short, there may be differences in the incidence rate of late seizures. Third, although there was no significant difference between the patients receiving prophylactic anticonvulsants and those patients without medication, the surgeon's decision regarding the prescription of prophylactic anticonvulsants may have acted as a selection bias. Fourth, in this study, valproate, levetiracetam, and phenytoin were administered by selection according to the preference of the surgeon. Therefore, it may be subject to systematic bias and influence the outcome of the analysis.
Cortical involvement may be a risk factor for seizure onset. The incidence of early seizures tended to decrease in patients prescribed prophylactic anticonvulsants. Further prospective, randomized, double-blinded trials are necessary. Moreover, additional studies on the effects of prophylactic anticonvulsants according to different types of medications should be conducted.
References
1. Arboix A, Comes E, Massons J, García L, Oliveres M. Relevance of early seizures for in-hospital mortality in acute cerebrovascular disease. Neurology. 1996; 47:1429–1435. PMID: 8960722.

2. Arboix A, García-Eroles L, Massons JB, Oliveres M, Comes E. Predictive factors of early seizures after acute cerebrovascular disease. Stroke. 1997; 28:1590–1594. PMID: 9259754.

3. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale : implications for stroke clinical trials : a literature review and synthesis. Stroke. 2007; 38:1091–1096. PMID: 17272767.

4. Berger AR, Lipton RB, Lesser ML, Lantos G, Portenoy RK. Early seizures following intracerebral hemorrhage : implications for therapy. Neurology. 1988; 38:1363–1365. PMID: 3412583.

5. Bladin CF, Alexandrov AV, Bellavance A, Bornstein N, Chambers B, Coté R, et al. Seizures after stroke : a prospective multicenter study. Arch Neurol. 2000; 57:1617–1622. PMID: 11074794.
6. Cheung CM, Tsoi TH, Au-Yeung M, Tang AS. Epileptic seizure after stroke in Chinese patients. J Neurol. 2003; 250:839–843. PMID: 12883927.

7. De Herdt V, Dumont F, Hénon H, Derambure P, Vonck K, Leys D, et al. Early seizures in intracerebral hemorrhage : incidence, associated factors, and outcome. Neurology. 2011; 77:1794–1800. PMID: 21975203.

8. De Reuck J, Hemelsoet D, Van Maele G. Seizures and epilepsy in patients with a spontaneous intracerebral haematoma. Clin Neurol Neurosurg. 2007; 109:501–504. PMID: 17509750.

9. Echlin FA, Arnett V, Zoll J. Paroxysmal high voltage discharges from isolated and partially isolated human and animal cerebral cortex. Electroencephalogr Clin Neurophysiol. 1952; 4:147–164. PMID: 13033793.

10. Garrett MC, Komotar RJ, Starke RM, Merkow MB, Otten ML, Connolly ES. Predictors of seizure onset after intracerebral hemorrhage and the role of long-term antiepileptic therapy. J Crit Care. 2009; 24:335–339. PMID: 19327321.

11. Giroud M, Gras P, Fayolle H, André N, Soichot P, Dumas R. Early seizures after acute stroke : a study of 1,640 cases. Epilepsia. 1994; 35:959–964. PMID: 7925167.

12. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Guidelines for epidemiologic studies on epilepsy. Epilepsia. 1993; 34:592–596. PMID: 8330566.
13. Gurjar M, Singhal S, Baronia AK, Azim A, Poddar B. Valproate-induced hyperammonemic encephalopathy : a reminder of rare complication of valproate. J Emerg Trauma Shock. 2011; 4:321–322. PMID: 21769226.

14. Kase CS, Williams JP, Wyatt DA, Mohr JP. Lobar intracerebral hematomas : clinical and CT analysis of 22 cases. Neurology. 1982; 32:1146–1150. PMID: 6889699.

15. Kaye AH, Andrewes D. Glasgow Outcome Scale : research scale or blunt instrument? Lancet. 2000; 356:1540–1541. PMID: 11075764.
16. Keene DL, Ventureyra EC. Hydrocephalus and epileptic seizures. Childs Nerv Syst. 1999; 15:158–162. PMID: 10361965.

17. Kilpatrick CJ, Davis SM, Tress BM, Rossiter SC, Hopper JL, Vandendriesen ML. Epileptic seizures in acute stroke. Arch Neurol. 1990; 47:157–160. PMID: 2302087.

18. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996; 27:1304–1305. PMID: 8711791.

19. Lipton RB, Berger AR, Lesser ML, Lantos G, Portenoy RK. Lobar vs thalamic and basal ganglion hemorrhage : clinical and radiographic features. J Neurol. 1987; 234:86–90. PMID: 3559644.

20. Morgenstern LB, Hemphill JC 3rd, Anderson C, Becker K, Broderick JP, Connolly ES Jr, et al. Guidelines for the management of spontaneous intracerebral hemorrhage : a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010; 41:2108–2129. PMID: 20651276.

21. Naidech AM, Garg RK, Liebling S, Levasseur K, Macken MP, Schuele SU, et al. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke. 2009; 40:3810–3815. PMID: 19797183.

22. Naidech AM, Kreiter KT, Janjua N, Ostapkovich N, Parra A, Commichau C, et al. Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke. 2005; 36:583–587. PMID: 15662039.

23. Passero S, Rocchi R, Rossi S, Ulivelli M, Vatti G. Seizures after spontaneous supratentorial intracerebral hemorrhage. Epilepsia. 2002; 43:1175–1180. PMID: 12366733.

24. Piatt JH Jr, Carlson CV. Hydrocephalus and epilepsy : an actuarial analysis. Neurosurgery. 1996; 39:722–727. discussion 727-728. PMID: 8880764.
25. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1981; 22:489–501. PMID: 6790275.
26. Reddig RT, Nixdorf KE, Jensen MB. The prophylactic use of an antiepileptic drug in intracerebral hemorrhage. Clin Neurol Neurosurg. 2011; 113:895–897. PMID: 21824722.

27. Sahaya K, Goyal MK, Sarwal A, Singh NN. Levetiracetam-induced thrombocytopenia among inpatients : a retrospective study. Epilepsia. 2010; 51:2492–2495. PMID: 21204814.

28. Sung CY, Chu NS. Epileptic seizures in intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 1989; 52:1273–1276. PMID: 2512371.

29. Treiman DM. Electroclinical features of status epilepticus. J Clin Neurophysiol. 1995; 12:343–362. PMID: 7560022.

30. Wang HC, Chang WN, Chang HW, Ho JT, Yang TM, Lin WC, et al. Factors predictive of outcome in posttraumatic seizures. J Trauma. 2008; 64:883–888. PMID: 18404052.

31. Weisberg LA, Shamsnia M, Elliott D. Seizures caused by nontraumatic parenchymal brain hemorrhages. Neurology. 1991; 41:1197–1199. PMID: 1866004.

33. Yang TM, Lin WC, Chang WN, Ho JT, Wang HC, Tsai NW, et al. Predictors and outcome of seizures after spontaneous intracerebral hemorrhage. Clinical article. J Neurosurg. 2009; 111:87–93. PMID: 19301969.

Fig. 1
A representative computed tomography scan on admission showing a deep intracerebral hemorrhage without intraventricular hemorrhage. Computed tomography of a 74-year-old male patient shows a 2.5×1.5 cm-sized intracerebral hemorrhage in left basal ganglia.
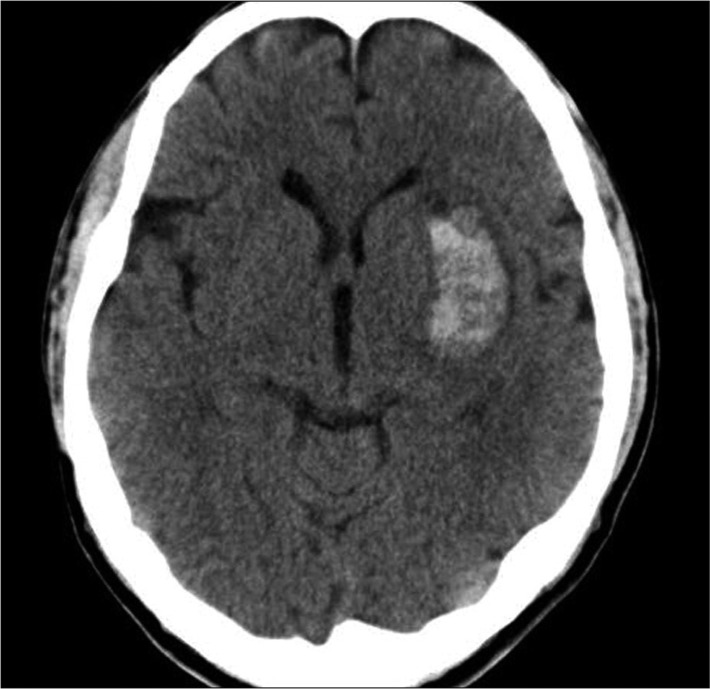
Fig. 2
A representative computed tomography scan on admission showing a deep intracerebral hemorrhage with intraventricular hemorrhage. Computed tomography of a 58-year-old male patient shows a 2×0.8 cm-sized intracerebral hemorrhage in left thalamus accompanied by intraventricular hemorrhage.
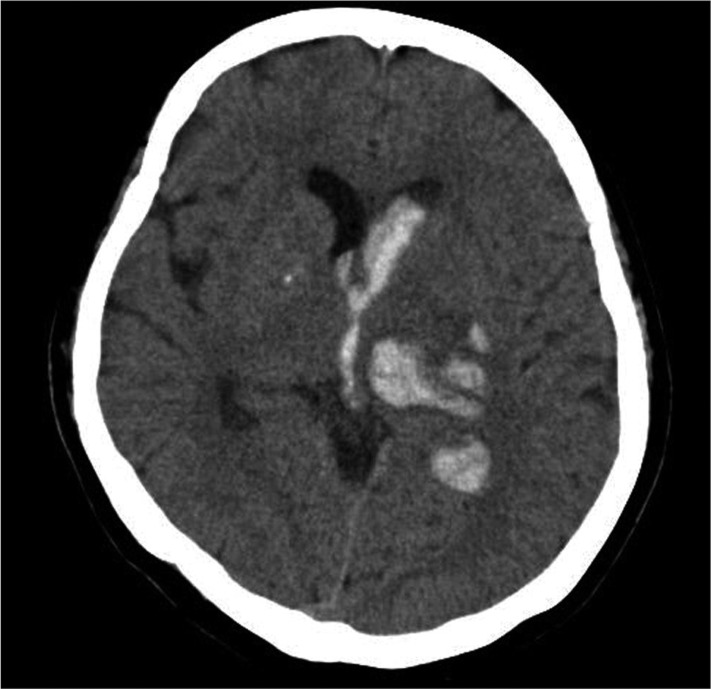
Fig. 3
A representative computed tomography scan on admission showing a lobar intracerebral hemorrhage without cortical involvement. Computed tomography of a 70-year-old male patient shows a 3.5×2 cm-sized intracerebral hemorrhage in right temporal lobe.
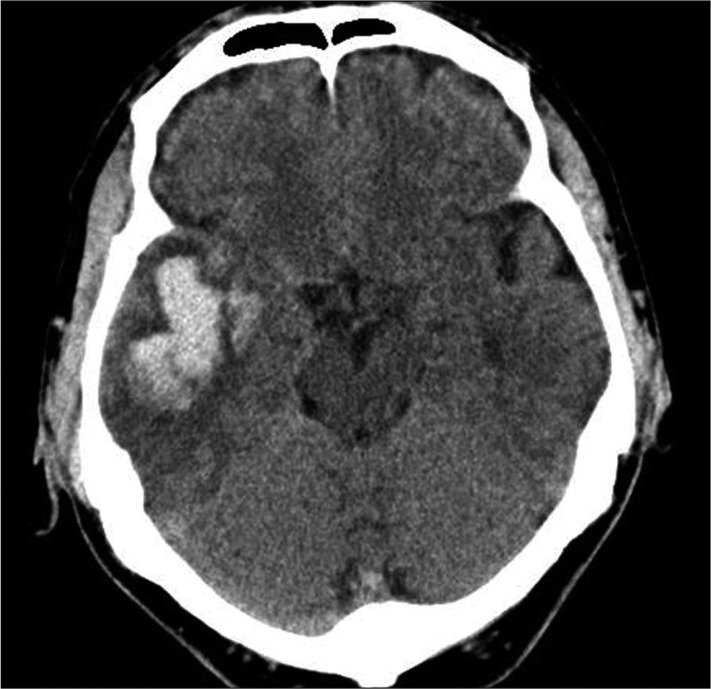
Fig. 4
A representative computed tomography of lobar intracerebral hemorrhage with cortical involvement. Computed tomography of a 64-year-old female patient shows a 3×2.5 cm-sized lobar intracerebral hemorrhage located in right parietal lobe.
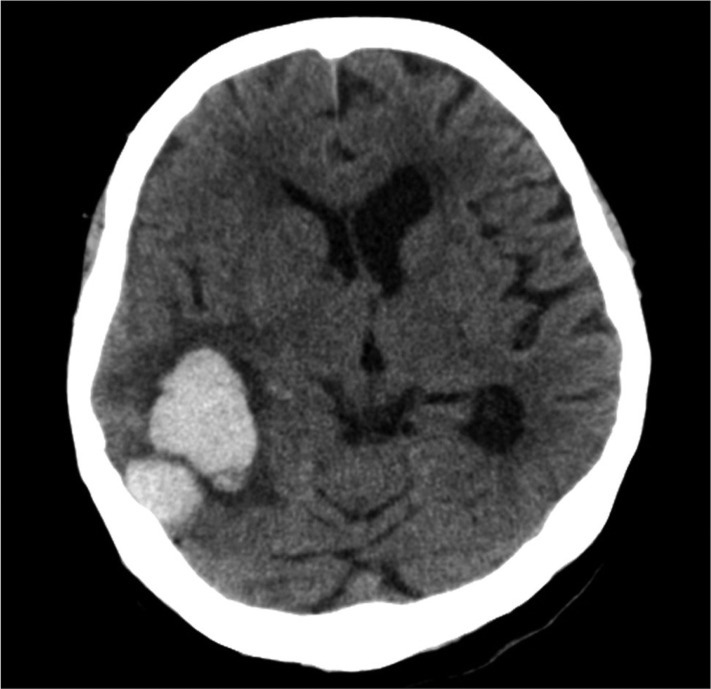
Table 1
Characteristics of the patients and details of ICH*
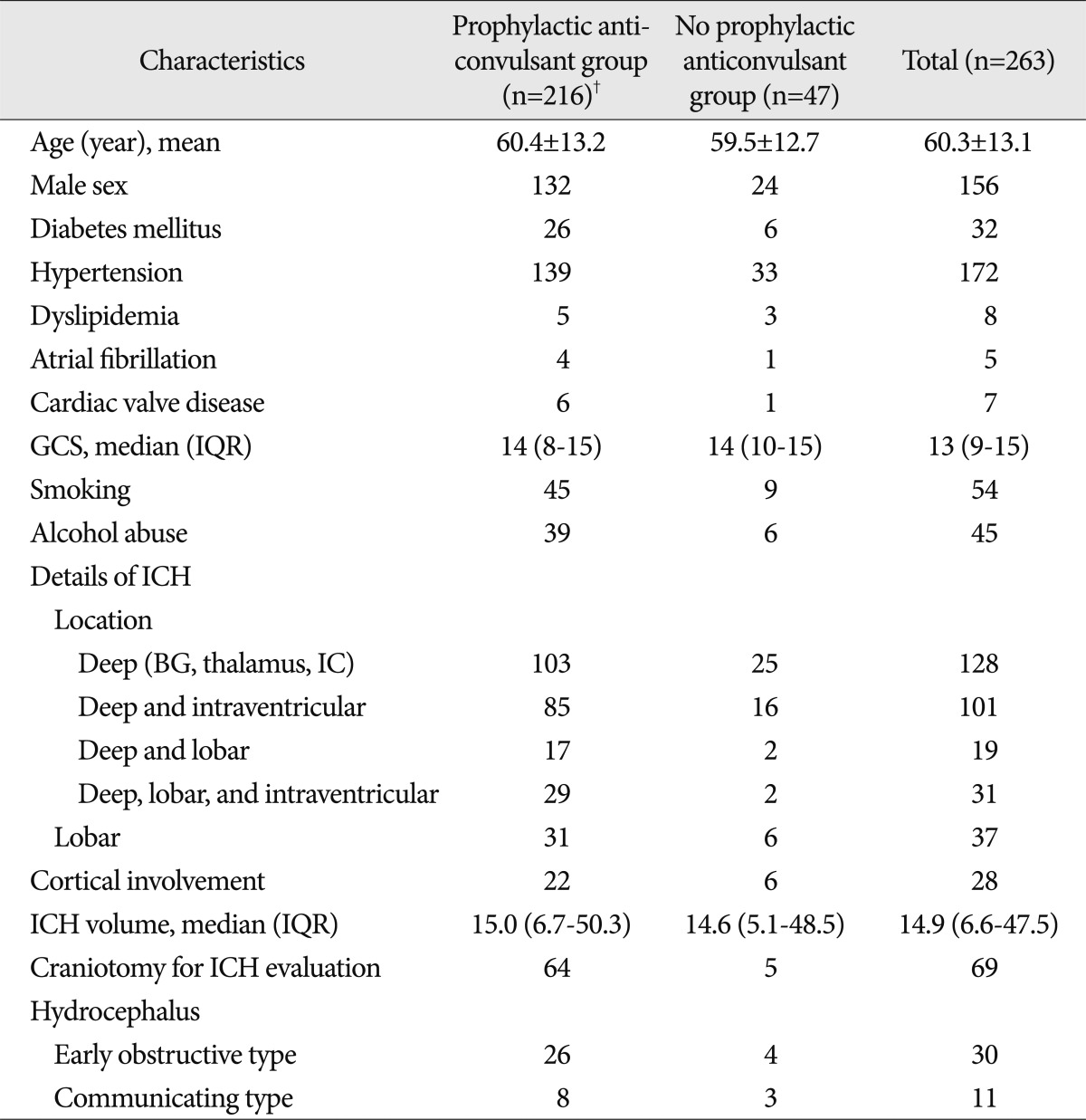
*The plus-minus values are the means±SDs, and the data are numbers of patients unless otherwise specified, †There was no significantly different parameter between the prophylactic anticonvulsant group (n=216) and the no prophylactic anticonvulsant group (n=47). BG : basal ganglia, IC : internal capsule, ICH : intracerebral hemorrhage, GCS : Glasgow Coma Scale, IQR : interquartile range




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download