Abstract
Spinal extradural arachnoid cysts usually cause symptoms related to spinal cord or nerve root compression. Here, we report an atypical presentation of a spinal extradural arachnoid cyst combined with congenital hemivertebra which was presented as a retroperitoneal mass that exerted mass effects to the abdominal organs. On image studies, the communication between the cystic pedicle and the spinal arachnoid space was indistinct. Based on our experience and the literature of the pathogenesis, we planned anterior approach for removal of the arachnoid cyst in order to focus on mass removal rather than ligation of the fistulous channel. In our estimation this was feasible considering radiologic findings and also essential for the symptom relief. The cyst was totally removed with the clogged 'thecal sac-side' end of the cystic pedicle. The patient was free of abdominal discomfort by one month after the surgery.
Extradural arachnoid cysts are uncommon enlarging lesions of the spinal cord which originate from small diverticula of the subarachnoid space3). Although termed "arachnoid cysts", the inner arachnoid lining has been shown to be frequently absent, and the term has been used interchangeably with "extradural meningeal cyst"9). Epidemiologically, these lesions demonstrate a male predominance and a peak incidence in the second decade of life1). These cysts occur most frequently in the mid to lower thoracic region, followed by the lumbosacral and thoracolumbar regions. Cervical extradural arachnoid cysts are relatively rare6).
Being a space-occupying lesion, the clinical presentation of an extradural arachnoid cyst is usually due to spinal cord and/or nerve root compression. Patients with thoracic cysts tend to suffer from spastic or flaccid paraparesis, while patients with lumbar and lumbosacral cysts complain of lower back pain, radiculopathy, or dysfunction of bladder and bowel2). Cervical cysts have been reported to cause spastic quadriparesis, and the Horner syndrome was observed to be associated with lesions at the lower cervical level2). In general, motor weakness predominates over sensory abnormalities6). Although symptoms manifest in a progressive manner, remission and fluctuation have been reported in 30% of cases2). Also, exacerbation of symptoms due to the Valsalva maneuver and the head-up position has been observed6).
We have encountered an unusual presentation of an extradural arachnoid cyst located in the retroperitoneal space. The patient complained of abdominal discomfort without any evidence of neurological compromise. The lesion was placed in retroperitoneal space without compression of neither spinal thecal sac nor nerve root.
A 26-year-old man presented with abdominal discomfort mainly over the right lower quadrant for 4 months. Although the patient did not have any significant medical or surgical past history, L4 hemivertebra had been incidentally found during a minor fall-off event in his teenage years (Fig. 1). He denied any symptom related to L4 hemivertebra including lower back pain and never had sought any treatment for such abnormality. In the examination, he did not complain any pain in his leg and lower back or any difficulty in defecation, voiding, and walking. We could not find any deficit in the neurologic examination, either.
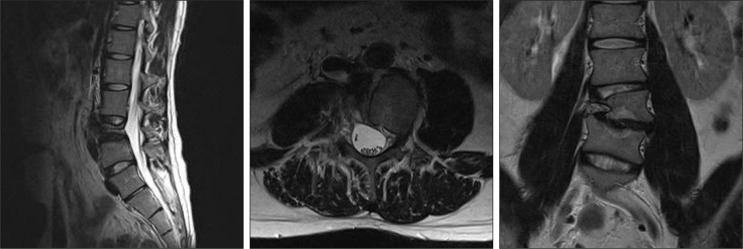
Symptoms of the patient were refractory to common digestive medicines and gastrointestinal motility modulating drugs. During the course of treatment, he underwent a series of studies, and a large cystic mass was first noted on abdominal sonography. For further diagnosis, he underwent abdominal computed tomography (CT) and lumbar spine magnetic resonance imaging (MRI), in turn. A large (6.2×4.0×8.0 cm) cystic mass originating from the right L4 spinal nerve root sleeve and extending to the prevertebral space of L2, L3, and L4 through the associated bony defect in the L4 vertebral body was revealed (Fig. 2). MRI demonstrated a homogenous signal, hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging that was not enhanced by gadolinium contrast media. On conventional lumbar MRI and magnetic resonance (MR) myelography, a long pedicle of the cyst was identified, but the communication between the cystic space and the spinal subarachnoid space was indistinct. In addition, the 'thecal sac-side' end of the cystic pedicle was cone-shaped and appeared to be clogged (Fig. 3).
Although in usual circumstances removal of a spinal extradural arachnoid cyst focuses on exploration and ligation of the fistulous channel1), a direct anterior retroperitoneal approach to the cyst was planned in this case, considering that removal of the main mass in the retroperitoneal space was essential for symptom relief, and that the communication between the cyst and the spinal subarachnoid space was unlikely. This decision was based on our experience with spinal extradural arachnoid cysts without identifiable communication points on imaging, in which detachment from the spinal cord was feasible and did not require ligation of the cystic origin. The literature also supported this concept of noncommunicating spinal extradural arachnoid cysts5,12).
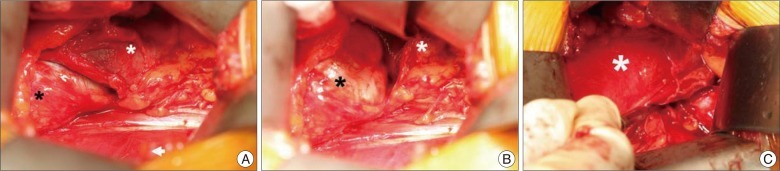
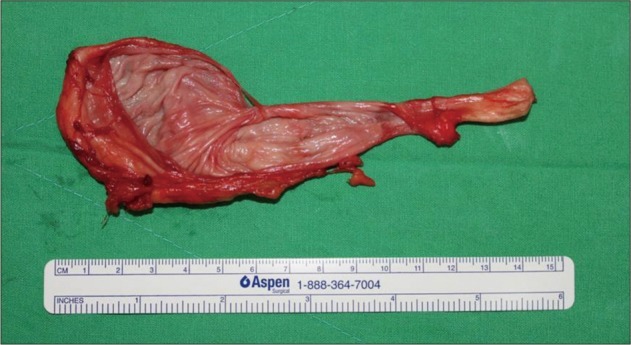
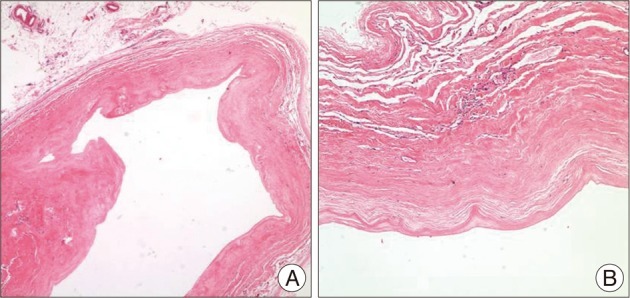
The patient was placed in a "three-quarter lateral" position, and an incision was made along the lateral margin of the rectus abdominis muscle. After retraction of peritoneum and dissection through the retroperitoneal fat, the right kidney was noted. The cyst was identified below the right kidney and was placed on the surface of psoas major muscle (Fig. 4). The cyst was displacing the inferior vena cava ventrally. For convenient dissection, internal decompression was performed; the cyst contained a clear liquid. The pedicle of the cyst originated from the intervertebral foramen, and the remaining cystic pedicle could be removed en bloc by undermining into the intervertebral foramen and gently drawing it. After the removal was completed we explored the cystic pedicle of the specimen with a blunt probe and observed that the 'thecal sac-side' end was clogged (Fig. 5). The final tissue pathology diagnosis was reported to be consistent with an arachnoid cyst (Fig. 6).
During the postoperative period, the patient did not complain of any symptoms other than postoperative pain. Flatus was passed on the first postoperative day, and the patient was discharged on the sixth postoperative day without any problem. A postoperative lumbar MRI was obtained one month after surgery and demonstrated no residual cyst and intact thecal sac without evidence of cerebrospinal fluid (CSF) leakage (Fig. 7). After the subsidence of postoperative pain, the patient was free of any abdominal discomfort.
Although the pathogenesis of spinal extradural arachnoid cysts has not yet been clarified, extradural arachnoid cysts are thought to be diverticula of the arachnoid membrane due to a dural defect which can be either congenital5) or acquired following
events such as spinal surgery, trauma, infection6,11), or percutaneous procedure7). The location of diverticula is known to be most commonly occurring at the junction of the theca and the nerve root sleeve followed by dorsal midline and the nerve root sleeve itself11).
At first the cysts must be merely small diverticula of arachnoid space and should get enlarged to cause any symptom. Several mechanisms have been postulated in order to explain the progressive nature of spinal extradural arachnoid cysts. Active fluid secretion from the lining cells of the cyst was proposed. But this could not explain observation that secretory cells were frequently absent in the lining3). Osmosis of water was also proposed considering xanthochromia of the cystic contents. The osmotic pressure of xanthochromic fluid is higher than that of tissue fluid3). More feasible explanation comes from the pulsatile nature of CSF. Intrathecal CSF dynamics change greatly by elevation of intra-abdominal pressure and this is far more influential than pressure change during the respiratory cycle. The change in intra-abdominal pressure result in enlargement of the cystic sac and persistent CSF pulsation cause continuous growth of the cyst under the law of Leplace11). One-way valvular mechanism was followed in order to complement the relationship between imposed hydrostatic pressure and continuous cystic growth. Folds of meninges at the ostium of the cyst can act as a flap-like one-way valve, or rather slit-like communication with the subarachnoid space results in one-way valve11). More recently, a 'ball-valve' theory was proposed to explain the valvular mechanism. When intrathecal pressure surges, the spinal arachnoid space is communicated with the cystic space and fluid flows into the cyst. As intrathecal pressure goes down, the cyst body exerts a force to impede the cystic pedicle following the law of Leplace as the cyst has the larger radius and therefore has the greater wall tension than the cystic pedicle. This is actually a two-way system of unequal flow, however, CSF is trapped in the cystic space6,11).
Various imaging modalities have been used to identify tissue communication points; however, CT myelography using water-soluble contrast media has been the study of choice for illuminating the location of communication points between the cyst and the spinal thecal sac6). More recently, there has been a report using cinematic MRI for detecting dural defects10). There have been cases in which the cyst was not filtrated by contrast media, or the pedicle was unidentifiable6). In our case, the patient has brought MR myelography from the referring hospital and refused additional CT myelography. The cystic pedicle and its communication with the spinal arachnoid space can be determined using MRI and MR myelography6).
To date, removal of extradural arachnoid cyst has focused on obliterating the fistulous channel, that is, the pedicle1). Choi et al.1) reported a case of spinal extradural arachnoid cyst which was removed following ligation of the fistulous channel, and reviewed 17 additional cases of spinal extradural arachnoid cyst which was either excised or ligated in a similar manner. Kulkarni et al.4) reported that, in their 7 cases of spinal extradural arachnoid cysts, they could not identify any connection between the cyst and the spinal arachnoid space in the operative field. In contrast, Cloward2) reported that communication was verified in 43 of 92 cases of congenital spinal extradural arachnoid cysts. Although there was no identifiable communication with the spinal arachnoid space, the characteristics of the cyst contents match that of CSF in the majority of cases4). McCrum and Williams8) explained the formation of these cysts without communication using osmosis or an active secretion mechanism previously mentioned.
It has been proposed that the communication between extradural arachnoid cysts and the subarachnoid space gradually decreases as the pressure gradient fades and eventually becomes nonexistent5). In other words, spinal extradural arachnoid cysts may or may not communicate with the spinal subarachnoid space depending on the stage of evolution. What can be deduced from this theory is that, if the communication is not detectable on imaging studies, the probability of communication disruption is high, so that, when deciding on the surgical plan, it is possible to disregard ligation of a fistulous channel in the cystic pedicle. In the current case, communication of the cystic pedicle with the spinal subarachnoid space was not detected on MR myelography, and the cystic pedicle was confirmed to be clogged.
This is an atypical presentation of a spinal extradural arachnoid cyst which extended into the retroperitoneal space, and was combined with a congenital vertebral malformation. The cystic pedicle was not communicating with the spinal arachnoid space on image study and the cyst was removed in en bloc manner with clogged 'thecal sac-side' end of the cystic pedicle by an anterior approach. This case supports the hypothesis that, when the cystic pedicle is not identified on image study, removal of the cyst without ligating the fistulous channel can be possibly done.
References
1. Choi JY, Kim SH, Lee WS, Sung KH. Spinal extradural arachnoid cyst. Acta Neurochir (Wien). 2006; 148:579–585. discussion 585. PMID: 16505968.

2. Cloward RB. Congenital spinal extradural cysts : case report with review of literature. Ann Surg. 1968; 168:851–864. PMID: 5684190.
3. Gortvai P. Extradural cysts of the spinal canal. J Neurol Neurosurg Psychiatry. 1963; 26:223–230. PMID: 13949360.

4. Kulkarni AG, Goel A, Thiruppathy SP, Desai K. Extradural arachnoid cysts : a study of seven cases. Br J Neurosurg. 2004; 18:484–488. PMID: 15799150.
5. Lake PA, Minckler J, Scanlan RL. Spinal epidural cyst : theories of pathogenesis. Case report. J Neurosurg. 1974; 40:774–778. PMID: 4826604.
6. Liu JK, Cole CD, Kan P, Schmidt MH. Spinal extradural arachnoid cysts : clinical, radiological, and surgical features. Neurosurg Focus. 2007; 22:E6. PMID: 17608349.
7. Mao HQ, Yang HL, Geng DC, Bao ZH, Tang TS. Spinal extradural arachnoid cyst following percutaneous vertebroplasty. Eur Spine J. 2011; 20(Suppl 2):S206–S210. PMID: 20835874.

8. McCrum C, Williams B. Spinal extradural arachnoid pouches. Report of two cases. J Neurosurg. 1982; 57:849–852. PMID: 7143073.
9. Nabors MW, Pait TG, Byrd EB, Karim NO, Davis DO, Kobrine AI, et al. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg. 1988; 68:366–377. PMID: 3343608.

10. Neo M, Koyama T, Sakamoto T, Fujibayashi S, Nakamura T. Detection of a dural defect by cinematic magnetic resonance imaging and its selective closure as a treatment for a spinal extradural arachnoid cyst. Spine (Phila Pa 1976). 2004; 29:E426–E430. PMID: 15454723.

11. Rohrer DC, Burchiel KJ, Gruber DP. Intraspinal extradural meningeal cyst demonstrating ball-valve mechanism of formation. Case report. J Neurosurg. 1993; 78:122–125. PMID: 8416228.

12. Sakellaridis N, Panagopoulos D, Mahera H. Sacral epidural noncommunicating arachnoid cyst. Case report and review of the literature. J Neurosurg Spine. 2007; 6:473–478. PMID: 17542517.
Fig. 1
Lumbar radiographs. A : Antero-posterior view showing L4 hemivertebra. B : Lateral view showing mild spondylolisthesis L4 on L5.
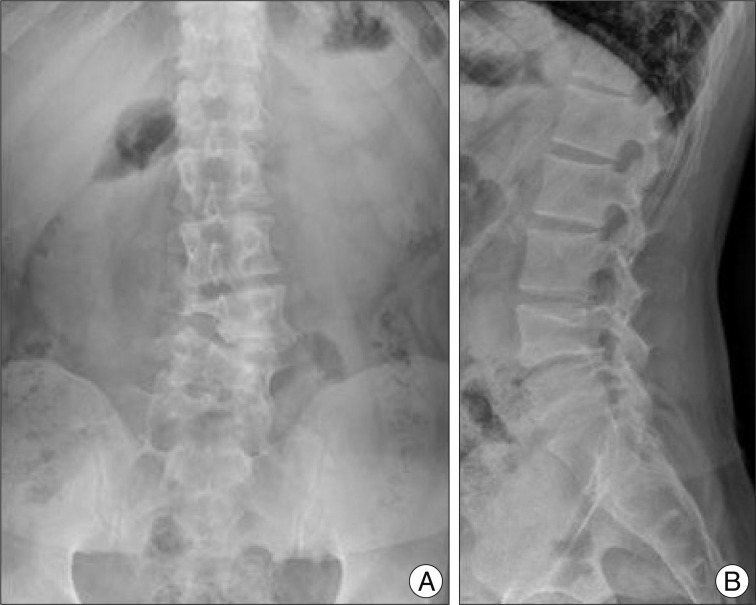
Fig. 2
Magnestic resonance myelography. A : Three dimensional view showing large cystic mass with a pedicle to the spinal thecal sac. B : Coronal view showing the end of cystic pedicle which is clogged.
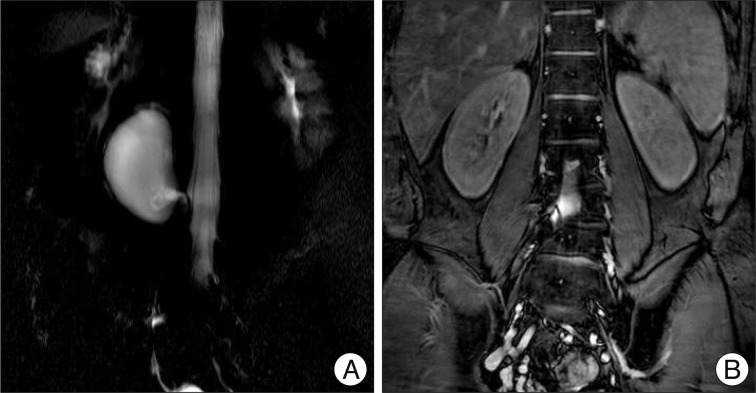
Fig. 3
T2-weighted magnetic resonance images. A : Sagittal view, images are in medial-to-lateral order. B : Axial view, images are in foot-to-head order going clockwise from the left upper image. Note the 'cone-shaped' cystic pedicle.
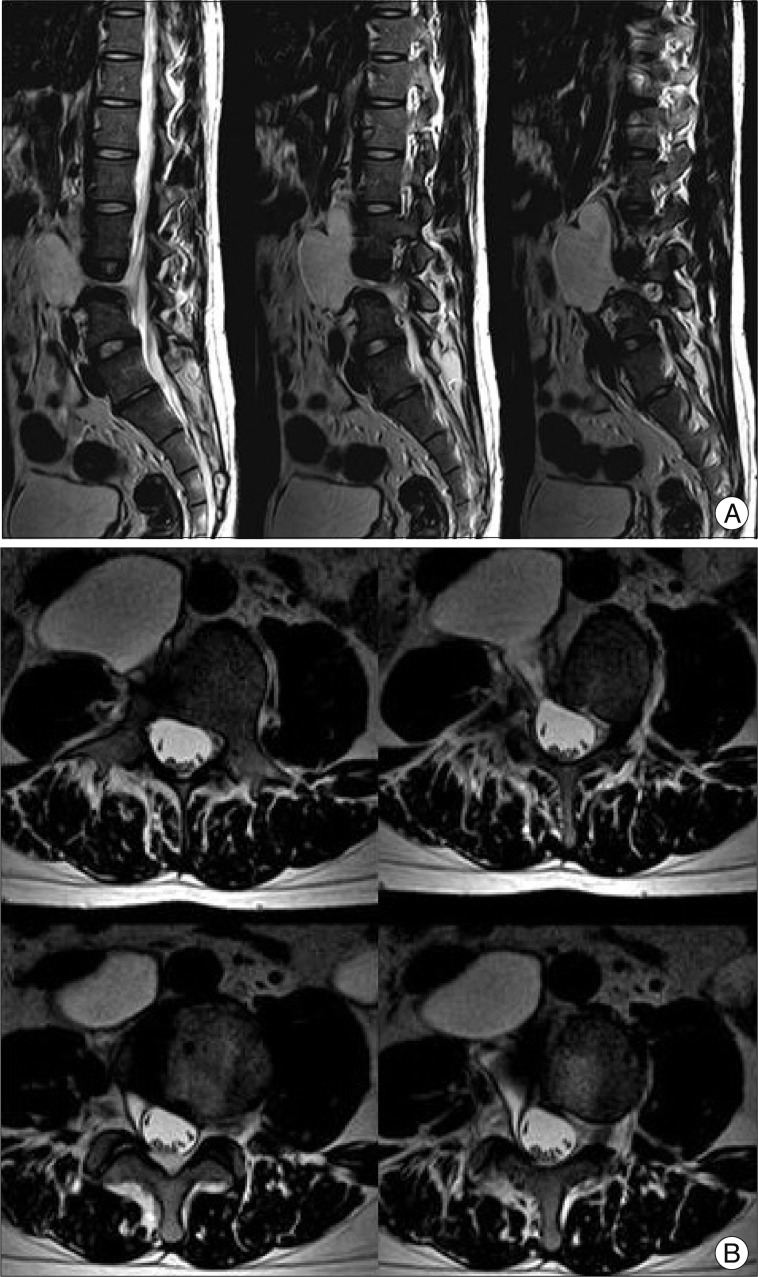
Fig. 4
Intraoperative view. A : Right kidney (white asterisk) and the arachnoid cyst (black asterisk) were exposed after the peritoneum (white arrow head) was retracted. B : Exposure of the arachnoid cyst after further retraction of right kidney. C : The arachnoid cyst was removed and the blunt dissector indicated the origin of the arachnoid cyst.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download