Abstract
Objective
The purpose of this prospective study was to evaluate the effects of body mass index (BMI) on intra-abdominal pressure (IAP) and intraoperative blood loss (IBL) during lumbar spinal surgery.
Methods
Thirty patients scheduled for single level posterior lumbar interbody fusion were allocated equally to a normal group (Group 1, BMI;18.5-22.9 kg/m2), an overweight group (Group 2, BMI; 23-24.9 kg/m2), and an obese group (Group 3, BMI; 25.0-29.9 kg/m2) according to BMI. IAP was measured using a urinary bladder catheter; 1) supine after anesthesia induction, 2) prone at skin incision, 3) prone at the end of surgery. In addition, IBL was also measured in the three groups.
Results
IAP in the supine position was not significantly different in groups 1, 2, and 3 (2.7 mm Hg, 3.0 mm Hg, and 4.2 mm Hg, respectively) (p=0.258), and IAP in the prone position at incision increased to 7.8 mm Hg, 8.2 mm Hg, and 10.4 mm Hg, respectively, in the three groups, and these intergroup differences were significant, especially for Group 3 (p=0.000). IAP at the end of surgery was slightly lower (7.0 mm Hg, 7.7 mm Hg, and 9.2 mm Hg, respectively). IBLs were not significantly different between the three groups. However, IBLs were found to increase with IAP in the prone position (p=0.022) and BMI (p<0.05).
Conclusion
These results show that BMI affects IAP in the prone position more than in the supine position during lumbar spinal surgery. In addition, IBLs were found to increase with IAP in the prone position and with BMI. Thus, IBLs can be expected to be higher in morbidly obese patients due to an increased IAP.
Spine surgeons are concerned about intraoperative bleeding during spinal surgery because even minor bleeding can obstruct the surgeon's field of vision8,12). In addition, significant blood loss during spinal surgery is associated with unfavorable surgical outcomes and prolonged hospital stays6,7). Furthermore, uncontrollable epidural bleeding is related to an increase in epidural venous system pressure. Thus, increased intra-abdominal pressure (IAP) due to abdominal compression in the prone position is transmitted to the inferior vena cava, and thus, to the epidural venous system, and increase bleeding2). In addition, epidural bleeding associated with increased IAP can be troublesome during posterior lumbar interbody fusion (PLIF) which requires bilateral radical discectomy and epidural vessel manipulation1,10).
In our hospital, a Wilson frame has been used exclusively used during spinal surgery. The Wilson frame provides a convenient and stable method of maintaining patients in a flexed position, and its two pads can be adjusted laterally to relieve pressure on the abdomen11). However, despite an appropriate pad width, the abdomen can be compressed excessively, and thus, increase epidural bleeding, especially in obese patients. Body mass index (BMI), defined as the individual's body weight divided by the square of his or her height, has been used as a parameter estimating obesity. Generally, BMI correlates with IAP4), and therefore, IAP is usually expected to be higher in obese patients. However, no study has addressed the degree to which BMIs affect IAP and blood loss during lumbar spinal surgery.
Accordingly, the purpose of this study was to evaluate the effect of BMI on IAP and IBL during lumbar spinal surgery.
This prospective study was conducted on a cohort of 30 patients classified as physical status I or II according to the American Society of Anesthesiologist classification, and scheduled for single level PLIF at L3-4, L4-5, or L5-S1. Our Institutional Human Investigation Ethic Committee approved the study, and written informed consent was obtained from all patients. Patients with hypertension, or a central nerve system, cardiac, liver, or renal disorder, or any contraindication to the placement of a transurethral bladder catheter were excluded. Patients with bleeding tendency and taking antiplatelet agents, such as, aspirin were also excluded. No patient had undergone previous spinal or abdominal surgery. The 30 patients were allocated equally to three groups according to World Health Organization guideline of BMI : Group 1, normal (BMI : 18.5-22.9 kg/m2), Group 2, overweight (BMI : 23-24.9 kg/m2), or Group 3, obese (BMI : 25.0-29.9 kg/m2)15). No patient was morbidly obese (BMI ≥30 kg/m2). All surgical procedures were performed by an experienced spine surgeon. Before positioning patients on the operating table, a Foley catheter was placed. IAP was measured in the following positions in all patients : 1) supine after induction, 2) prone on a Wilson frame at skin incision, and 3) prone at the end of surgery before repositioning.
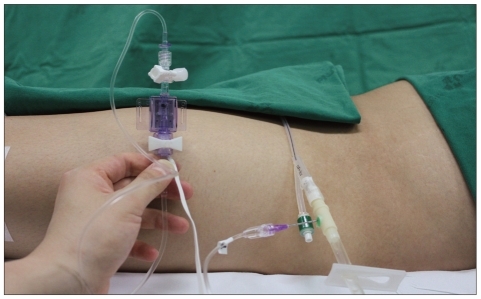
Due to the invasiveness of direct IAP measurements, we measured IAP indirectly by using a Foley catheter to measure urinary bladder pressure. Because the bladder wall acts as a passive diaphragm when the bladder volume is between 50 and 100 mL in adults, urinary bladder pressure can be used as an index of IAP5,9). Urinary bladder pressure was measured as described by Kron et al.9) (Fig. 1), as follows.
1) The bladder was emptied after Foley catheter placement.
2) 50 mL of sterile saline was injected into the bladder through the catheter.
3) The catheter was then connected to a urinary bladder bag.
4) Air in the drainage catheter was eliminated by filling the catheter with fluid from the bladder.
5) After clamping the drainage bag tubing just downstream of the connection site, an 18-gauge needle was inserted through the catheter sampling port and connected to a pressure transducer.
6) The symphysis pubis was used as the reference point of transducer and pressure was recorded at the end of the expiratory phase.
All surgical procedures were performed by one spine surgeon. The patient was placed into the prone position on the Wilson frame after general anesthesia. The distance between lateral borders of frame pads was adjusted according to patient's shoulders width. The pads was enough flexed for better access of discectomy, preparing the disc space and endplate, and inserting interbody cages. The abdomen was allowed to hang freely, as much as possible, to avoid abdominal wall tension. Female breasts were positioned in a comfortable, natural position.
A midline skin incision was then made, and paraspinous musculatures were dissected laterally to the base of transverse processes using subperiosteal dissectors. Spinous processes were removed using a rongeur. Subtotal laminectomy, facetectomy, and foraminotomy were performed to decompress dural sac and nerve roots completely. The laminar bone chips obtained during posterior decompression were prepared for impact into cages. Before discectomy, the epidural venous plexus was carefully cauterized with a bipolar coagulator. Traditional bilateral radical discectomy was performed following incision of the annulus. The end plate was completely prepared using an angled curette and a round curette. Two carbon fiber cages impacted with lamina bone chips were inserted into the disc space. Residual disc space was filled with remnant lamina bone chips and allobone chips. Transpedicular screw fixation was performed after inserting cages. Bleeding was meticulously controlled and the wound was irrigated copiously with saline. Finally, a Hemo-Vac drain was inserted and the wound was closed in a layer-by-layer manner.
IBL was estimated by measuring blood contents in the suction bottle and weighing blood-soaked gauzes. The blood contents in the suction bottle were measured by subtracting irrigating saline volume from the volume of contents in the suction bottle. In addition, the blood contents in gauzes and pads were measured by subtracting the weights of pure gauzes from the total weight of blood-soaked gauzes.
One-way ANOVA and the trend test were used to determine the significances of age, sex, and operative time differences between the three groups, and one-way ANOVA was used to assess intergroup differences with respect to the relationship between IAP and IBL. In addition, linear regression (SPSS version 12.0 for Windows, SPSS Inc., Chicago, IL, USA) was used identify correlations between BMI, IAP, and IBL. Statistical significance was accepted for p values of <0.05.
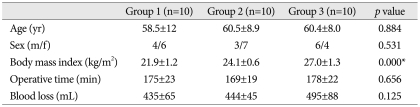
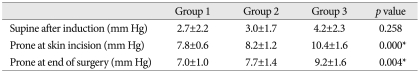
No significant intergroup differences were observed for age, sex, or operating time (Table 1). IAP in the supine position was 2.7±2.2 mm Hg in Group 1, 3.0±1.7 mm Hg in Group 2, and 4.2±2.3 mm Hg in Group 3 (p=0.258). IAPs in the prone position on the Wilson frame at incision increased to 7.8±0.6 mm Hg, 8.2±1.2 mm Hg, and 10.4±1.6 mm Hg, respectively, in the three groups (p=0.000), whereas IAPs in the prone position on the Wilson frame at end of surgery was slightly lower at 7.0±1.0 mm Hg, 7.7±1.4 mm Hg, and 9.2±1.6 mm Hg, respectively (p=0.004) (Table 2). IAPs in the supine position were not significantly different in the 3 groups, but IAPs in prone position on the Wilson frame were significantly different. In particular, IAP was significantly higher in the obese group.
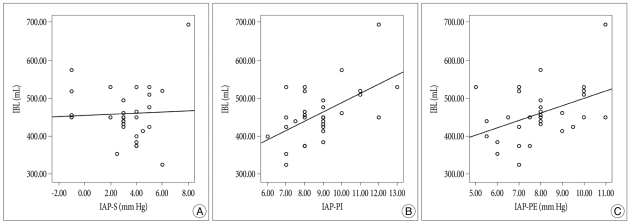
IBL was 435±65 mL in Group 1, 444±45 mL in Group 2, and 495±88 mL in Group 3 (p=0.125) (Table 1), which were not significantly different. However, the correlation between BMI and IBL in all 30 patients was statistically significant (p=0.022) (Fig. 2), as was the correlation between the two in the prone position (p<0.05). On the other hand, the correlation between IBL and IAP in the supine position was not significant (p=0.092) (Fig. 3).
Intraoperative bleeding is one of the main concerns of spine surgeons during spinal surgery, because bleeding can obscure the surgical field and because greater than estimated blood loss can cause perioperative complications and result in unfavorable surgical outcomes6-8,12). Intraoperative bleeding is probably associated with factors, such as, surgeons' skill and surgical position. However, bone bleeding due to osteoporosis can also increase total blood loss during lumbar surgery. In particular, an increased IAP due to abdominal compression in prone position has been reported to be a cause of excessive intraoperative blood loss because it increases vertebral venous pressure11). Therefore, many efforts have been made to avoid abdominal compression in the prone position. Böstman et al.3) reported that mean blood loss during lumbar discectomy is much reduced when patients are operated on in the kneeling position rather than in the prone position. Sundén et al.14) showed that epidural venous pressure and blood loss are less for a negative pressure cushion than a Wilson frame. However, these authors did not measure IAP, which provides an objective measure of abdominal compression in surgical position.
Park11) first measured IAP changes in patients undergoing lumbar surgery on a Wilson frame, and it was found that IAP and IBL can be reduced by adjusting the pad width. However, IAP was measured using rectal pressure, which is less reliable than urinary bladder pressure. In recent studies, urinary bladder pressure has been viewed as the golden standard indicator of IAP5,9). Rigamonti et al.12) first used urinary bladder pressure to measure IAP during lumbar surgery, and found that IAP did not differ between the prone position on a modified Relton-Hall frame and the knee-chest position on an Andrew-type table. In addition, no correlation was found between IBL and IAP. However, the IBL was evaluated based on the surgeons' assessment of degree of visual field impairment, and no volumetric evaluation of IBL was performed.
Despite the efforts made to reduce abdominal compression and IAP, the prone position on a Wilson frame is widely used because of its convenience and because it provides a flexed position for spinal surgery11). We hypothesized that in the optimal prone position on the Wilson frame, abdominal compression and intraoperative bleeding could be more troublesome in obese or overweight patients.
This is the first prospective study to show the effect of BMI on IAP and IBL during lumbar spinal surgery. Urinary bladder pressure was used as a proxy of IAP, and contrary to previous studies, our cohort was limited to patients undergoing single level PLIF, because the amount of blood loss during minor surgeries like microdiscectomy has little affect on patient outcome. In this study, IAP in the supine position was within the normal range of 2.7 to 4.2 mm Hg and no significant difference was found between mean group IAPs. However, after prone positioning on the Wilson frame, IAPs in groups 1, 2, and 3 increased to 7.8, 8.2, and 10.4 mm Hg, respectively and IAP were significantly different in the three groups. In particular, the IAP of obese patients was significantly higher. This result indicates that BMI affect IAP in the prone position more so than in the supine position during lumbar surgery. However, in the present study, at the end of surgery, mean IAP was slightly lower than at the beginning of surgery. This slight reduction in IAP at the end of surgery is at odds with the observations of Rigamonti et al.12), who found a slight increase in IAP at the end of surgery. We speculated that the redistribution of intra-abdominal contents or fat may have caused the mild decrease observed in the present study. However, the slight IAP differences observed are of marginal interest only. The relationship between IAP and BMI has been previously investigated by Sanchez et al.13), who found a mean IAP of 6.5 mm Hg in patients with a mean BMI of 27.6 kg/m2, and that IAP was significantly related to BMI and recent abdominal surgery. Cobb et al.4) reported that the IAPs of healthy adults were correlated with BMIs, especially, during standing and coughing. However, no previous report has addressed the relationship between BMI and IAP in the prone position during spinal surgery.
In the present study, IBL was not significantly different in the three groups, but blood loss increased in proportion to IAP in the prone position. This relation may be because single level PLIF is not major enough to affect blood loss in patients with a BMI of <30.0 kg/m2. In addition, meticulous bleeding control by the operator could have minimized IBL differences. Nevertheless, IBL increased in proportion to IAP and BMI in the prone position, but not in the supine position. These finding suggest that blood loss can be expected to be substantially higher in the morbidly obese. However, no morbidly obese patient was included because of the rarity of single level PLIF in such patients at our hospital.
This study has several limitations that warrant consideration. First, the cohort size was small because few patients satisfied the inclusion criteria and because of the prospective study designed. Second, IAP was not measured in morbidly obese patients with a BMI of >30.0 kg/m2. Third, parameters estimating obesity are various. Central obesity can affect much IAP and IBL during spinal surgery. Fourth, IAP and blood loss were only measured in prone position on the Wilson frame, and the use of the Jackson surgical table, which has been optimized for spinal surgery, is increasing. Nevertheless, we believe that our findings are meaningful, because they are the first results published of relations between BMI and IAP and resultant blood loss during lumbar surgery.
This prospective study describes the effects of BMI on IAP and IBL during lumbar spinal surgery. Mean IAPs in the supine position were similar in the three study groups, but mean IAPs in the prone position differed, and mean IAP was particularly high in the obese group. The result implies that BMI can more affect IAP in the prone position than in the supine position during lumbar surgery. Furthermore, although, mean IBLs during single level PLIF were non-significantly different in the three groups, IBL was found to increase in proportion to IAP and BMI in prone position, but not in the supine position. Thus, our findings predict that IBL is likely to be substantially higher for morbidly obese patients undergoing lumbar spinal surgery.
Acknowledgements
This study was supported by Biomedical Research Institute Grant (2010-11), Pusan National University Hospital.
References
1. Aono H, Ohwada T, Hosono N, Tobimatsu H, Ariga K, Fuji T, et al. Incidence of postoperative symptomatic epidural hematoma in spinal decompression surgery. J Neurosurg Spine. 2011; 15:202–205. PMID: 21529204.

2. Batson OV. The function of the vertebral veins and their role in the spread of metastases. 1940. Clin Orthop Relat Res. 1995; 4–9. PMID: 7634616.
3. Böstman O, Hyrkäs J, Hirvensalo E, Kallio E. Blood loss, operating time, and positioning of the patient in lumbar disc surgery. Spine (Phila Pa 1976). 1990; 15:360–363. PMID: 2363066.

4. Cobb WS, Burns JM, Kercher KW, Matthews BD, James Norton H, Todd Heniford B. Normal intraabdominal pressure in healthy adults. J Surg Res. 2005; 129:231–235. PMID: 16140336.

5. Fusco MA, Martin RS, Chang MC. Estimation of intra-abdominal pressure by bladder pressure measurement : validity and methodology. J Trauma. 2001; 50:297–302. PMID: 11242295.

6. Imagama S, Kawakami N, Tsuji T, Ohara T, Matsubara Y, Kanemura T, et al. Perioperative complications and adverse events after lumbar spinal surgery : evaluation of 1012 operations at a single center. J Orthop Sci. 2011; 16:510–515. PMID: 21725670.

7. Karikari IO, Isaacs RE. Minimally invasive transforaminal lumbar interbody fusion : a review of techniques and outcomes. Spine (Phila Pa 1976). 2010; 35:S294–S301. PMID: 21160393.
8. Kou J, Fischgrund J, Biddinger A, Herkowitz H. Risk factors for spinal epidural hematoma after spinal surgery. Spine (Phila Pa 1976). 2002; 27:1670–1673. PMID: 12163731.

9. Kron IL, Harman PK, Nolan SP. The measurement of intra-abdominal pressure as a criterion for abdominal re-exploration. Ann Surg. 1984; 199:28–30. PMID: 6691728.

10. Lin PM. A technical modification of Cloward's posterior lumbar interbody fusion. Neurosurgery. 1977; 1:118–124. PMID: 355915.

11. Park CK. The effect of patient positioning on intraabdominal pressure and blood loss in spinal surgery. Anesth Analg. 2000; 91:552–557. PMID: 10960374.

12. Rigamonti A, Gemma M, Rocca A, Messina M, Bignami E, Beretta L. Prone versus knee-chest position for microdiscectomy : a prospective randomized study of intra-abdominal pressure and intraoperative bleeding. Spine (Phila Pa 1976). 2005; 30:1918–1923. PMID: 16135980.

13. Sanchez NC, Tenofsky PL, Dort JM, Shen LY, Helmer SD, Smith RS. What is normal intra-abdominal pressure? Am Surg. 2001; 67:243–248. PMID: 11270882.
14. Sundén G, Wallöe A, Wingstrand H. A new device to reduce intra-abdominal pressure during lumbar surgery. Spine (Phila Pa 1976). 1986; 11:635–636. PMID: 3787332.

15. WHO/IASO/IOTF. The Asia-Pacific perspective : redefining obesity and its treatment. 2000. Melbourne: Health Communications Australia;ISBN 0-9577082-1.
Fig. 2
Correlation of intraoperative blood loss (IBL) with body mass index (BMI). There was a significant correlation (p=0.022).
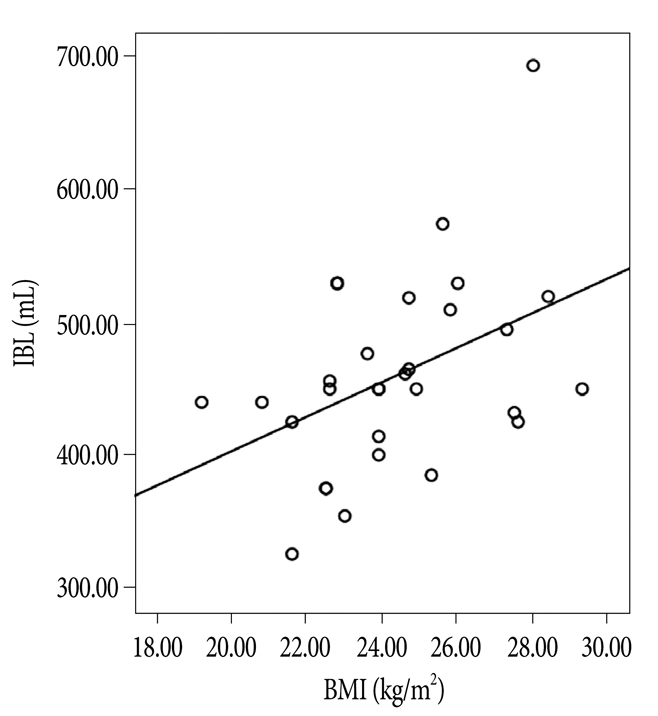
Fig. 3
Correlation of intraoperative blood loss (IBL) with intra-abdominal pressure in supine (IAP-S) (A), IAP in prone at the incision (IAP-PI) (B), and IAP in prone at the end of surgery (IAP-PE) (C). There was no correlation between IBL and IAP-S (p=0.092), but there was significant correlation between IBL and IAP in prone (p<0.05).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download