Abstract
Internal carotid artery (ICA) trapping can be used for the treatment of giant intracranial aneurysms, blood blister-like aneurysms, and fusiform dissecting aneurysms. Fusiform dissecting aneurysms are challenging to treat surgically and endovascularly because of no definite neck and critical perforators. Surgical or endovascular trapping of the ICA with or without an extracranial-intracranial bypass has commonly been used as an effective method to treat these lesions, but balloon test occlusion (BTO) must be performed. Here, we report a case of a ruptured fusiform dissecting aneurysm of the distal ICA, which was successfully treated using an endovascular ICA trapping with a manual ICA compression test instead of BTO.
Intracranial fusiform aneurysms account for about 1% of cerebral aneurysms, are caused by atherosclerosis, and usually develop in the basilar artery, internal carotid artery (ICA), or middle cerebral artery (MCA)2,10). The management of ruptured fusiform aneurysm remains controversial. These lesions are managed with surgical or endovascular ICA trapping with or without bypass. However, it is necessary to undergo balloon test occlusion (BTO) for ICA trapping.
Here, we present an interesting case of a ruptured fusiform dissecting aneurysm of the distal ICA, which was successfully treated using endovascular ICA trapping with a manual ICA compression test instead of BTO for ICA occlusion.
A 77-year-old woman presented with sudden onset of a severe headache. Her condition was assessed as Hunt and Hess Grade III and Fisher Grade IV. A computed tomography (CT) scan and CT angiography revealed two aneurysms, one at the right ICA and the other at the left MCA bifurcation. There was no subarachnoid hemorrhage (SAH) at the left sylvian fissure (Fig. 1A).
Conventional cerebral angiography demonstrated a fusiform dissecting aneurysm involving the right distal ICA (Fig. 1B) whose long axis was 10 mm in length and a saccular aneurysm at the left middle cerebral artery (Fig. 1C). Collateral flow via the anterior communicating artery (ACoA) from the contralateral ICA was noted through an ipsilateral ICA compression test. A manual ICA compression test showed a 1.5-second venous drainage delay in the ipsilateral ICA (Fig. 2). The patient was planned to undergo BTO for ICA occlusion surgically or endovascularly. But, BTO was not possible due to the clinically critical and unstable condition of the patient and decrease her mental status. Also, we could not prolong clinical test with BTO under local anesthesia. We had to perform any treatments rapidly and decided to do an endovascular treatment. Subsequently, general anesthesia was applied and then, bilateral femoral punctures were performed, and two 6F guiding catheters (Envoy MPC 90 Cordis; Johnson and Johnson Medical, Miami Lakes, FL, USA) were positioned at each ICA under the general anesthesia. No further ICA occlusion test was performed. The right catheter was for stenting and/or trapping, and the one on the left was for angiography. Double stents (4.5×30 mm, 4.5×20 mm; Neuroform stents, Boston Scientific/Target, Fremont, CA, USA) were deployed at the right distal ICA to diverse a flow and inhibit coil protrusion via the aneurysm. After stent placement, it was not sufficient to diverse a flow and trapping was performed at the aneurysm site. Repeat angiography confirmed complete trapping of the fusiform dissecting aneurysm. Collateral flow via the ACoA from the contralateral ICA showed no venous phase delay. The patient regained consciousness one day after the operation, and no focal neurological deficits. She started daily oral antiplatelet therapy with aspirin 100 mg and clopidogrel 75 mg. Three days after the operation, the patient developed mild dysarthria and grade IV weakness of the left hand. Diffusion-weighted imaging (DWI) showed an acute infarction at the right internal capsule and multiple embolic infarctions in the right MCA territory (Fig. 3C). We immediately started heparinization and continued it for one week. The patient recovered completely and was discharged five weeks later.
Fusiform aneurysms present with a spindle-shaped appearance and are characterized by dilated and tortuous arteries associated with atherosclerosis2). These aneurysms develop from the parent artery, irrespective of any bifurcation, forming a broad base, and both the parent artery and the aneurysm exhibit marked atherosclerosis2).
As a fusiform dissecting aneurysm of the distal ICA is a rare pathologic condition, the clinical features remain unclear. Recently, associated dissection has been proposed in the pathogenesis of blood blister-like aneurysms of the ICA8,16). Fusiform aneurysms are usually associated with ischemia or neural compression and rarely present as SAH with rupture2). The etiology of these aneurysms remains unknown. Both underlying atherosclerotic disease and recurrent arterial dissections secondary to hypertension have been implicated as causes of pseudoaneurysms of a fusiform architecture15,17).
Fusiform dissecting aneurysms are challenging to manage surgically and endovascularly because of the lack of a defined neck that can be used to distinguish the aneurysm from the parent artery. Also, critical perforating vessels frequently originate directly from the parent artery, and the location of the aneurysm is often nonamenable to a surgical approach17). These characteristics of fusiform aneurysms result in high surgical morbidity and mortality rates, and the risk of aneurysm regrowth and rebleeding after surgery remains high despite the availability of various surgical strategies, such as clip placement, wrapping, clip placement on wrapped material, and ICA trapping4,14).
Recently, endovascular managements, such as coil embolization or intracranial stent placement, have been attempted for fusiform dissecting aneurysms. However, these lesions are difficult to treat with conventional endovascular techniques because they have no neck. Higashida et al.7) first reported the use of stent-assisted coil embolization for a fusiform dissecting aneurysm at the basilar artery. Also, Mericle et al.11) reported a successful result with stent-assisted coil embolization to treat wide-neck pseudoaneurysm arising from a narrowed petrous carotid artery. But, we think that endovascular coil embolization cannot be recommended in this case of ruptured fusiform dissecting aneurysm because the risk of procedural rupture is too high. Therefore, we planned to diverse the flow by double stenting. However, double stenting was not sufficient to occlude pseudoaneurysm, thus ICA trapping using detachable coils were performed.
Surgical trapping of the ICA with or without an extracranial-intracranial (EC-IC) bypass has commonly been used as an effective method for the treatment of giant intracranial aneurysms, blood blister-like aneurysms, and ruptured fusiform dissecting aneurysms5,9). Endovascular ICA trapping is an alternative method for these lesions. If there is sufficient collateral flow from the posterior communicating arteries (PCoA) or ACoA, patients will tolerate the procedure without requiring EC-IC bypass surgery after surgical or endovascular trapping of the ICA5).
However, test occlusion must be performed to evaluate ischemic risks and to reduce the complication rate of ischemia before surgical or endovascular trapping/occlusion of the ICA is performed. Many BTO protocols have been designed to increase the sensitivity of the clinical occlusion test and to reduce the significant risk of delayed ischemic events. Most of the methods propose continuing balloon inflation for 15 to 120 minutes under local anesthesia13). If the occlusion time is prolonged, it may increase the risk of the thromboembolic complications, even when full anticoagulation with heparin is performed1).
Abud et al.1) proposed the temporary occlusion test that is based on the symmetry of the venous drainage delay in BTO of the ICA. Their findings suggest that patients with a delay in the venous drainage less than 2 seconds in the BTO can safely undergo ICA occlusion, and this corresponds to a negative cerebral blood flow (CBF) test. A venous drainage delay of 2-4 seconds probably corresponds to a borderline CBF evaluation, and it is difficult to evaluate the incidence of complications. Finally, more than 4 seconds of venous drainage delay probably corresponds to a poor CBF test. These patients are at high risk for the development of complications after permanent ICA occlusion. BTO of the ICA based on the analysis of venous symmetry is a safe, reliable, and simple procedure to induce permanent occlusion of the ICA. This procedure can reduce the prolonged time of balloon inflation and, as a result, lower thromboembolic risk. In our case, it was necessary to perform BTO for trapping of the ICA. However, it was difficult to perform BTO for a long time because of the patient's poor mental status and rebleeding risk before general anesthesia. Also, it is necessary to perform a systemic heparinization for prolonged BTO. However, it is unadvisable to do that in patients with SAH because of rebleeding risk. Access for balloon inflation can be difficult to patients with critical states. Repeat balloon inflation and deflation in unstable state can be occurred to intimal injury without anesthesia. We performed an occlusion test of the ICA using manual compression instead of balloon inflation because we needed to decided if endovascular trapping was possible before applying general anesthesia in angiosuite. Generally, manual compression test is not a standard method instead of BTO. However, we think that this could be an alternative to BTO in some situations. Hetzel et al.6) reported that the manual carotid compression test with transcranial Doppler sonography to exclude patients at high risk, is a useful tool for testing tolerance prior to invasive testing. In our case, the compression test revealed a venous phase delay of 1.5 seconds. We think that a manual compression test may have some disadvantages. A manual compression in elderly patients with atherosclerotic change can induce an embolic risk. Moreover, CCA trauma can occur. It can be difficult to identify ICA flow that is completely occluded.
The cardinal arteries, such as the ophthalmic artery, PCoA, and anterior choroidal artery (AChA), require consideration during ICA trapping12). The PCoA should often be occluded together with the lesions. However, if a fetal type or large PCoA is included in the lesion segment, other therapeutic strategies should first be considered. Clinically, the AChA is the most important branch to be saved. The origin of the AChA is often too close to the lesion to be occluded. If the AChA originates from the lesion segment, alternative treatments, such as clip placement or wrapping, should be considered12). In addition, endovascular occlusion at the focal ICA segment is technically difficult. Unlike aneurysm clips, the precise and stable placement of endovascular devices, such as coils and balloons, is difficult.
In our case, there was involvement of the whole communicating segment of the ICA and no defined neck to exclude from the parent artery. Moreover, critical perforating vessels, such as the AChA and PCoA, frequently originate directly from the aneurysm. The location of the aneurysm often presents a challenge to the surgical approach. We performed a stent-in-stent technique to change the intra-aneurysmal flow dynamics and to promote healing and thrombus formation within the aneurysmal sac. But, it was not enough to obtain immediate occlusion of the pseudoaneurysmal sac. Therefore, ICA trapping was completed via coil packing. As the coil packing progressed, the flow of the AChA slowed. Finally, the AChA was not visible on conventional angiography during the final coil trial (Fig. 3A). Fortunately, repeat angiography revealed a recanalized AChA, and we discovered complete occlusion of the ICA (Fig. 3B). Although it was uncertain how the AChA became recanalized, we speculate that a fine flow entered the AChA during the remodeling between the coil and the strut of the open-cell type stent. We administrated aspirin 100 mg and clopidogrel 75 mg after the operation. DWI showed multiple embolic infarctions on the right side (Fig. 3C). The patient showed no neurological deficits immediately after the operation but developed grade IV left sided weakness three days later. Possibility of vasospasm was considered due to SAH because of there was infarction at right deep borderzone and symptoms were developed at 3 days after procedure. Magnetic resonance angiography, however, revealed no definite vasospasm of major vessels except an artifact by coil mass and transcranial Doppler was normal range. We believed the cause was the thromboembolism by coil trapping and delayed circulation by cross-filling through ACoA. Therefore, we maintained heparinization for one week, and the patient was discharged without neurological deficits after five weeks. Of course, episodes of early or late aneurysm rebleeding can occur in patients treated with coil embolization who has residual filling of the neck or body of the aneurysm in acute stage. However, Bernardini et al.3) reported that anticoagulation with intravenous heparin was given to patients after embolization with complete or incomplete obliteration of the aneurysm lumen for a mean duration of 6 days. They demonstrated that heparinization does not appear to increase the risk of early rebleeding after coil embolization in patients suffered from SAH. After 40 days, the previous finding of multiple embolic infarctions was not observed on MRI. Complete occlusion of the ICA and ipsilateral blood flow with no delay through the ACoA were seen on conventional angiography. Also, the AChA was saved (Fig. 3D).
Treatment for a ruptured fusiform dissecting aneurysm of the ICA remains controversial. Stent-assisted coil occlusion can prevent rerupture during the acute stage. For permanent ICA occlusion, BTO of the ICA based on an analysis of the symmetry of the venous phase is a safe, reliable, and simple procedure can reduce the prolonged balloon inflation with possible lower thromboembolic risk. But, as our case shows, manual compression test is likely to an alternative method to BTO in some limited cases.
References
1. Abud DG, Spelle L, Piotin M, Mounayer C, Vanzin JR, Moret J. Venous phase timing during balloon test occlusion as a criterion for permanent internal carotid artery sacrifice. AJNR Am J Neuroradiol. 2005; 26:2602–2609. PMID: 16286409.
2. Aoki Y, Nemoto M, Yokota K, Kano T, Goto S, Sugo N. Ruptured fusiform aneurysm of the proximal anterior cerebral artery (A1 segment). Neurol Med Chir (Tokyo). 2007; 47:351–355. PMID: 17721050.

3. Bernardini GL, Mayer SA, Kossoff SB, Hacein-Bey L, Solomon RA, Pile-Spellman J. Anticoagulation and induced hypertension after endovascular treatment for ruptured intracranial aneurysms. Crit Care Med. 2001; 29:641–644. PMID: 11373436.

4. Charbel FT, Gonzales-Portillo G, Hoffman W, Cochran E. Distal internal carotid artery pseudoaneurysms : technique and pitfalls of surgical management : two technical case reports. Neurosurgery. 1999; 45:643–648. discussion 648-649. PMID: 10493387.
5. Chung JH, Shin YS, Lim YC, Park M. Ideal internal carotid artery trapping technique without bypass in a patient with insufficient collateral flow. J Korean Neurosurg Soc. 2009; 45:260–263. PMID: 19444357.

6. Hetzel A, von Reutern G, Wernz MG, Droste DW, Schumacher M. The carotid compression test for therapeutic occlusion of the internal carotid artery. Comparison of angiography with transcranial Doppler sonography. Cerebrovasc Dis. 2000; 10:194–199. PMID: 10773645.

7. Higashida RT, Smith W, Gress D, Urwin R, Dowd CF, Balousek PA, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case report and review of the literature. J Neurosurg. 1997; 87:944–949. PMID: 9384409.

8. Islam MS, Manabe H, Hasegawa S, Takemura A, Nagahata M. Successful staged treatment for ruptured blister-like dissecting aneurysm of the intracranial internal carotid artery : acute GDC embolization for the blister-like aneurysm followed by proximal occlusion with extracranial-intracranial bypass in the chronic stage. Minim Invasive Neurosurg. 2004; 47:165–168. PMID: 15343433.

9. Lawton MT, Hamilton MG, Morcos JJ, Spetzler RF. Revascularization and aneurysm surgery : current techniques, indications, and outcome. Neurosurgery. 1996; 38:83–92. discussion 92-94. PMID: 8747955.
10. Little JR, St Louis P, Weinstein M, Dohn DF. Giant fusiform aneurysm of the cerebral arteries. Stroke. 1981; 12:183–188. PMID: 7233461.

11. Mericle RA, Lanzino G, Wakhloo AK, Guterman LR, Hopkins LN. Stenting and secondary coiling of intracranial internal carotid artery aneurysm : technical case report. Neurosurgery. 1998; 43:1229–1234. PMID: 9802870.
12. Park JH, Park IS, Han DH, Kim SH, Oh CW, Kim JE, et al. Endovascular treatment of blood blister-like aneurysms of the internal carotid artery. J Neurosurg. 2007; 106:812–819. PMID: 17542524.

13. Peterman SB, Taylor A Jr, Hoffman JC Jr. Improved detection of cerebral hypoperfusion with internal carotid balloon test occlusion and 99mTc-HMPAO cerebral perfusion SPECT imaging. AJNR Am J Neuroradiol. 1991; 12:1035–1041. PMID: 1763721.
14. Redekop GJ, Woodhurst B. Unusual aneurysms of the distal internal carotid artery. Can J Neurol Sci. 1998; 25:202–208. PMID: 9706721.

15. Sakata N, Takebayashi S, Kojima M, Masawa N, Suzuki K, Takatama M, et al. Different roles of arteriosclerosis in the rupture of intracranial dissecting aneurysms. Histopathology. 2001; 38:325–337. PMID: 11318898.

16. Shigeta H, Kyoshima K, Nakagawa F, Kobayashi S. Dorsal internal caendovascular rotid artery aneurysms with special reference to angiographic presentation and surgical management. Acta Neurochir (Wien). 1992; 119:42–48. PMID: 1481751.

17. Wakhloo AK, Mandell J, Gounis MJ, Brooks C, Linfante I, Winer J, et al. Stent-assisted reconstructive endovascular repair of cranial fusiform atherosclerotic and dissecting aneurysms : long-term clinical and angiographic follow-up. Stroke. 2008; 39:3288–3296. PMID: 18772450.

Fig. 1
Initial computed tomography (A) shows diffuse subarachnoid hemorrhage, except left sylvian fissure. Conventional cerebral angiography demonstrats a fusiform dissecting aneurysm involving the right distal internal carotid artery (B) and a bilobulated saccular aneurysm at the middle cerebral artery bifurcation (C).
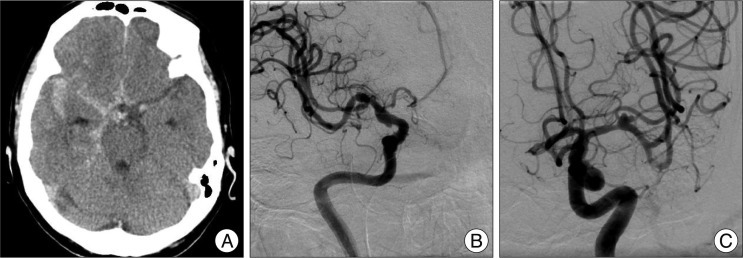
Fig. 2
Anteroposterior view of the conventional angiogram demonstrating a right carotid manual compression test in a patient with a ruptured fusiform dissecting aneurysm. The late arterial phase demonstrating good cross-filling through anterior communicating artery (A and B). One second after the previous image B, there are still veins on the left side and the beginning of venous phase on the right side (C). In an image taken 0.5 seconds later, the venous phase synchronously becomes more evident in both hemispheres (D). This patient had a 1.5-second venous phase delay, and permanent occlusion was performed.
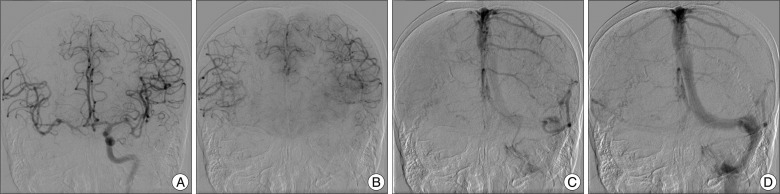
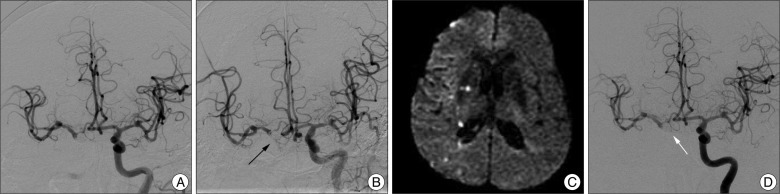
Fig. 3
Conventional angiogram (A) that was performed during coil trapping revealed that the anterior choroidal artery (AChA) had disappeared. Fortunately, follow-up angiography (B) in the immediate postoperative period demonstrates the recanalized AChA (black arrow). Three days later, the patient had mild dysarthria and grade IV left hand weakness. Diffusion-weighted MR image (C) shows multiple embolic infarctions of the right hemisphere in the territory of middle cerebral artery within the right internal capsule. We administrated heparinization for one week, and the symptoms were improved at discharge. Follow-up angiography (D) 40 days later reveals good collateral circulation through the anterior communicating artery and the right AChA is saved (white arrow).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download