Abstract
Hemorrhagic complications associated with aspirin use occur primarily at skin or gastrointestinal sites but can occasionally occur in the central nervous system. In particular, spontaneous spinal epidural hemorrhage (SSEH) associated with aspirin is very rare. We report a case of low-dose (100 mg daily) aspirin-related SSEH that was successfully treated with medical management. Our case indicates that low-dose aspirin could induce SSEH and that conservative treatment with close observation and repeated imaging studies should be considered in cases with neurological improvement or mild deficits.
Spontaneous spinal epidural hematoma (SSEH) is rare; however, it can present with neurological status change ranging from radiculopathy to complete quadriplegia depending on the severity of compression4). These hematomas have been reported in association with coagulopathies, anticoagulant therapy, tumor, infection, pregnancy and vascular malformations4,7-9). However, no definite cause is found in the majority of cases4). Among these potential causative factors, anticoagulants may seem to be predictable16), but SSEH associated with anti-platelet agents is very rare. There are only six reported cases of aspirin-related SSEH1,14,18,20,26,27). In particular, the cases of SSEH related with low-dose aspirin are only two26,27). Here, we present a case of SSEH related with low-dose aspirin that was successfully treated without surgery.
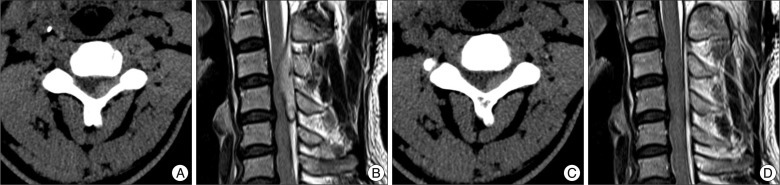
A 50-year-old man visited the emergency room complaining of severe neck pain and quadriparesis [American Spinal Injury Association (ASIA) impairment scale : C]. He had no history of trauma or spinal pain. He had been taking aspirin (100 mg daily) without medical advice or prescription for four months. General laboratory findings were normal, but platelet aggregation time was slightly prolonged. Bleeding time (Collagen/Epinephrine clotting time) was 230 sec, which was slightly delayed (normal range : 81-192 sec). Initial spine computerized tomography (CT) showed a high-density mass in the dorsal portion of the epidural space from C3 to C5 (Fig. 1A). He was prescribed a neck brace and steroid therapy (dexamethasone 5 mg IV) with a prophylactic antacid agent. One hour later, partial improvement was observed in his neurological status (ASIA impairment scale : C→D), and we were able to safely obtain a spinal magnetic resonance image (MRI). MRI showed a large hematoma in the dorsal portion of the epidural space from C3 to C5, causing spinal cord compression (Fig. 1B). Three hours later, the patient demonstrated normal motor function with mild numbness in both arms. Therefore, we continued conservative treatment (brace and steroid) in the intensive care unit and performed a follow-up CT. Follow-up CT one day later showed a partial decrease in the hematoma (Fig. 1C), and his neurological deficits had fully recovered. Spinal artery digital substraction angiography was performed to rule out vascular lesion, and there was no abnormality. Seven days later, a follow-up MRI showed complete resolution of the epidural hematoma (Fig. 1D) and enhanced MR images showed no abnormal enhancing lesion, such as tumor or vascular malformation. The patient was discharged without neurological deficit.
SSEH is a relatively rare disease : its incidence is 0.1 patients per 100000, representing <1% of spinal epidural space occupying lesions17). The main causes include vascular malformation, anticoagulant therapy, and neoplasm7,8,13,20,26). Of these potential causative factors, anticoagulants may be predictable : it has been reported that 25-70% of SSEH patients have a history of anticoagulant use16). However, SSEH associated with anti-platelet agents is very rare.
Anti-platelet drug is frequently used in various diseases, and their hemorrhagic complications usually occur at skin or gastrointestinal sites15,19). In particular, aspirin is one of the most widely used medications in the world, with approximately 40000 ton consumed each year27). Aspirin (also known as acetylsalicylic acid) is used for its analgesic, antipyretic, and anti-inflammatory properties. Aspirin also has antiplatelet effects due to inhibition of thromboxane production; thus, it is used long-term at low doses to help prevent heart attacks, ischemic strokes, and blood clot formation in patients at high risk for clot development15). However, there are also well-established undesirable side effects of aspirin, including stomach bleeding, gastrointestinal ulcers, tinnitus, and hemorrhagic stroke especially at higher doses15,19).
There have been six reported cases of aspirin-related SSEH1,14,18,20,26,28) and only two cases of low-dose aspirin-related SSEH26,28). The CURRENT-OASIS 7 study reported that occurrences of major bleeding, such as gastrointestinal bleeding and intracranial hemorrhage, did not differ between high-dose (300-325 mg daily) and low-dose aspirin (75-100 mg daily)19). Wagner et al.26) reported low-dose aspirin induced SSEH, which showed moderate coagulopathy including of 10-16% platelet aggregation with arachidonic acid and collagen (normal range : >70%) and normal bleeding time. In our case, platelet aggregation and bleeding time were slightly delayed, so we cannot completely rule out the possibility of a coincidence of the medical history of aspirin intake and SSEH. However, significant numbers of the patients with secondary intracranial hemorrhage related with low-dose aspirin showed normal or mild abnormal platelet function and bleeding time20,21,25). Although the SSEH is different from the intracranial hemorrhage, but the probability of aspirin-related SSEH would seem to be higher considering its action mechanism and patient's radiologic and lab findings.
Immediate surgical decompression of the neural structure is the treatment of choice in the majority of patients, but conservative management has occasionally been considered11,12). Decision to treat conservatively is based on the neurological status and surgical condition of the patient. Neurological improvement and mild neurological deficit without progression are good indications for conservative treatment12,17). Coexisting serious coagulopathy and/or anticipated risks of operative treatment may also be relative indications for conservative treatment10,23). However, there are some debates regarding the methods of conservative treatment.
Some authors suggested that immediate replacement therapy in patients with a coagulopathy prevents progression of the hematoma, allowing for the improvement of neurological signs and symptoms without operation10,24). In contrast, Connolly et al.5) stated that coagulopathy-induced spinal bleeds are amendable to conservative treatment because the hematoma remains liquid for a longer time compared to that in cases with normal clotting, therefore enabling the spread of the hematoma into the spinal epidural space. Crabbe suggested that immobilization of the neck and administration of steroids may result in rapid improvement of neurological deficits in cervical SSEH6), and Fukui et al.9) reported that cervical/cervicothoracic localization favors spontaneous recovery after SSEH.
However, it is clear that intensive neurologic examination and follow-up imaging studies are necessary during conservative treatment. In our case, we chose neck immobilization and steroid administration, which was shown to have a rapid response and excellent outcome. Recovery or dramatic improvement of clinical symptoms without surgery is not common, although there are some reports1,3,5,11,24,26). We performed follow-up CT one day later and MRI seven days later. Although his neurological deficits had fully recovered after one day, we need a follow-up imaging study for precise care. MRI is the best diagnostic modality11), but it requires a longer study time and has a high cost compared to CT. CT is also a good follow-up modality1), so we performed a follow-up CT after one day.
There has been much speculation on the cause of spontaneous resolution of neurological signs and symptoms after SSEH. Among the various theories, the widely known "spreading theory" which suggests spread of the hematoma within the spinal canal along the spinal epidural space, is a plausible explanation for spontaneous recovery11,12). Groen suggested that spread of a hematoma remains possible until blood clotting is complete and that delayed clot formation (due to anticoagulants or coagulopathies) may promote spreading of the hematoma2,11).
In our case, the neurological deficits had fully recovered at 24 hours; by the seventh day, follow-up MRI showed complete resolution of the epidural hematoma. Wagner et al. reported a case of aspirin-related SSEH that had completely resolved on day 3 as seen on MRI. These rapid recoveries are meaningful considering that other reports have noted recovery times of six days to two months2,11). Although there have been only two cases of rapid resolutions, they may be explained by Groen's description2,11).
The use of aspirin in Korea continues to become more widespread with the increase in the elderly population22). Although we could not find clinical data regarding the use of low-dose aspirin in Korea, it is believed to be widely used. Our case indicates that low-dose aspirin can induce SSEH and physicians should be aware of this rare but serious complication. Also, conservative treatment involving close observation and repeated imaging studies may be considered in those with neurological improvements or mild deficits.
To our knowledge, this is the first Korean case of SSEH related with low-dose aspirin that was successfully treated without surgical treatment.
References
1. Anderson TJ, Donaldson IM. Spontaneous resolution of cervical spinal epidural haematoma. Postgrad Med J. 1989; 65:488–490. PMID: 2602243.

2. Boukobza M, Guichard JP, Boissonet M, George B, Reizine D, Gelbert F, et al. Spinal epidural haematoma : report of 11 cases and review of the literature. Neuroradiology. 1994; 36:456–459. PMID: 7991091.

3. Brawn LA, Bergval UE, Davies-Jones GA. Spontaneous spinal epidural haematoma with spontaneous resolution. Postgrad Med J. 1986; 62:885–887. PMID: 3809086.

4. Bruyn GW, Bosma NJ. Vin-ken PJ, Bruyn GW, editors. Spinal extradural haematoma. Handbook of Clinical Neurology. 1976. Vol 26. Amsterdam: North-Holland;p. 1–30.
5. Connolly ES Jr, Winfree CJ, McCormick PC. Management of spinal epidural hematoma after tissue plasminogen activator. A case report. Spine (Phila Pa 1976). 1996; 21:1694–1698. PMID: 8839474.

6. Crabbe DC, Mendelow AD, Pharoh P, Large DM, Ions GK. Cervical spinal extradural haematoma causing a transient Brown-Sequard syndrome. J Neurol Neurosurg Psychiatry. 1992; 55:239. PMID: 1564497.

7. Foo D. Spinal epidural hematoma. J Neurosurg. 1996; 84:308. PMID: 8592245.
8. Foo D, Chang YC, Rossier AB. Spontaneous cervical epidural hemorrhage, anterior cord syndrome, and familial vascular malformation. Neurology. 1980; 30:1253–1254. PMID: 7191526.

9. Fukui MB, Swarnkar AS, Williams RL. Acute spontaneous spinal epidural hematomas. AJNR Am J Neuroradiol. 1999; 20:1365–1372. PMID: 10472999.
10. García López A, Pérez Lara JM, Herrainz Hidalgo R, Puente Gonzalo E. Spinal epidural hematoma following thrombolytic therapy for acute myocardial infarction. Orthopedics. 1999; 22:987–988. PMID: 10535565.

11. Groen RJ. Non-operative treatment of spontaneous spinal epidural hematomas : a review of the literature and a comparison with operative cases. Acta Neurochir (Wien). 2004; 146:103–110. PMID: 14963742.

12. Halim TA, Nigam V, Tandon V, Chhabra HS. Spontaneous cervical epidural hematoma : report of a case managed conservatively. Indian J Orthop. 2008; 42:357–359. PMID: 19753167.

13. Harris DJ, Fornasier VL, Livingston KE. Hemangiopericytoma of the spinal canal. Report of three cases. J Neurosurg. 1978; 49:914–920. PMID: 731310.
14. Heye N. Is there a link between acute spinal epidural hematoma and aspirin? Spine (Phila Pa 1976). 1995; 20:1931–1932. PMID: 8560344.

15. Lewis HD Jr, Davis JW, Archibald DG, Steinke WE, Smitherman TC, Doherty JE 3rd, et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1983; 309:396–403. PMID: 6135989.

16. Liao CC, Lee ST, Hsu WC, Chen LR, Lui TN, Lee SC. Experience in the surgical management of spontaneous spinal epidural hematoma. J Neurosurg. 2004; 100:38–45. PMID: 14748572.

17. Liu Z, Jiao Q, Xu J, Wang X, Li S, You C. Spontaneous spinal epidural hematoma : analysis of 23 cases. Surg Neurol. 2008; 69:253–260. discussion 260. PMID: 17900669.
18. Locke GE, Giorgio AJ, Biggers SL Jr, Johnson AP, Salem F. Acute spinal epidural hematoma secondary to aspirin-induced prolonged bleeding. Surg Neurol. 1976; 5:293–296. PMID: 1265647.
19. Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7) : a randomised factorial trial. Lancet. 2010; 376:1233–1243. PMID: 20817281.

20. Mishima K, Aritake K, Morita A, Miyagawa N, Segawa H, Sano K. [A case of acute spinal epidural hematoma in a patient with antiplatelet therapy]. No Shinkei Geka. 1989; 17:849–853. PMID: 2797370.
21. Ohm C, Mina A, Howells G, Bair H, Bendick P. Effects of antiplatelet agents on outcomes for elderly patients with traumatic intracranial hemorrhage. J Trauma. 2005; 58:518–522. PMID: 15761345.

22. Park IB, Kim DJ, Kim J, Kim H, Kim H, Min KW, et al. Current status of aspirin user in Korean diabetic patients using Korean health insurance database. J Korean Diabetes Assoc. 2006; 30:363–371.

23. Rois PV, López MR, de Vergara BC, de la LamaZaragoza A, García JG, Uxo JM. Spinal epidural hematoma in hemophilic children : controversies in management. Childs Nerv Syst. 2009; 25:987–991. discussion 993, 995. PMID: 19360421.

24. Schmitz A, Wallny T, Sommer T, Brackmann H, Schulze-Bertelsbeck D, Effenberger W, et al. Spinal epidural haematoma in haemophilia A. Haemophilia. 1998; 4:51–55. PMID: 9873866.
25. Tauber M, Koller H, Moroder P, Hitzl W, Resch H. Secondary intracraroidnial hemorrhage after mild head injury in patients with low-dose acetylsalicylate acid prophylaxis. J Trauma. 2009; 67:521–525. discussion 525. PMID: 19741394.

26. Wagner S, Forsting M, Hacke W. Spontaneous resolution of a large spinal epidural hematoma : case report. Neurosurgery. 1996; 38:816–818. PMID: 8692404.
27. Warner TD, Mitchell JA. Cyclooxygenase-3 (COX-3) : filling in the gaps toward a COX continuum? Proc Natl Acad Sci USA. 2002; 99:13371–13373. PMID: 12374850.
28. Weber J, Hoch A, Kilisek L, Spring A. [Spontaneous intraspinal epidural hematoma secondary to use of platelet aggregation inhibitors]. Dtsch Med Wochenschr. 2001; 126:876–878. PMID: 11569370.

Fig. 1
Initial and follow-up imaging studies. A : Initial cervical CT shows a high density mass in the dorsal portion of the epidural space from C3 to C5. B : Initial T2 sagittal cervical MRI shows a huge mass in the dorsal portion of the epidural space from C3 to C5 with cord compression. C : Twenty-four hour follow-up CT shows a partial decrease of the hematoma. D : Seven day follow-up MRI (T2 sagittal image) shows complete resolution of the epidural hematoma.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download