Abstract
Many reports have been published on complications related to middle cerebral artery (MCA) aneurysm surgical clipping procedures. We report an emergency intracranial in situ bypass surgery case which was performed as a rescue procedure after aneurysmal neck laceration during clipping of an MCA large aneurysm. In this case, we performed in situ M3-superficial temporal artery (STA) interposition graft-M3 bypass procedure. If a STA-MCA anastomosis is not available under MCA flow obstruction, we can consider an emergency in situ MCA-MCA bypass procedure with or without an STA interposition graft.
Unruptured middle cerebral artery (MCA) aneurysms can be optimally treated by surgical clipping and endovascular coiling. Many complications related to the surgical clipping procedure have been reported. We report an emergency intracranial in situ bypass surgery case which was performed as a rescue procedure after aneurysmal neck laceration during MCA large aneurysm clipping.
A 76-year-old woman presenting with a one-month standing headache visited our outpatient clinic. On neurological examinations, no focal neurological deficits were observed. Outside brain magnetic resonance imaging revealed two unruptured intracranial aneurysms: a right MCA aneurysm and a distal A2-3 aneurysm. Aneurysm size was measured by transfemoral carotid angiography (TFCA). The MCA aneurysm measured 11.3×9.0 mm, and the A2-3 aneurysm measured 7.1×3.1 mm (Fig. 1). A right MCA aneurysm clipping operation was planned because of the headache location (right temporal area) and aneurysmal size (the MCA aneurysm was larger than the A2-3 aneurysm).
During the clipping procedure, neck laceration of the MCA aneurysm occurred due to severe atherosclerosis of the aneurysm. The lacerated length was too long to use a clipping technique with cotton or Bemsheet material coverage. Consequently, primary closure of the aneurysmal neck was performed with 8-0 nylon, and correct re-clipping was done. The temporary trapping time during primary closure was fourteen minutes, and the waves on the motor evoked potential (MEP) and somatosensory evoked potential (SSEP) decreased after eight minutes of trapping (Fig. 2). Moreover, the flow of the inferior M2 trunk was not detected after primary closure of aneurysmal neck, and the MEP/SSEP change was not recovered. Thus, we planned to perform emergency bypass surgery, in situ M2-M2 side-to-side anastomosis as a rescue procedure, because the parietal branch of the superficial temporal artery (STA) was sacrificed when the skin incision was made. But, it was not possible to perform in situ M2-M2 bypass, because of limited mobility (cannot be fully pulled to be sutured). In addition, limited vessel mobility also disturbed in situ M3-M3 bypass, so we planned to do M3-STA interposition graft-M3 anastomosis with harvesting the short pedicle of the STA frontal branch.
After harvesting the frontal branch of the STA, the first anastomosis between the M3 of the inferior M2 trunk and the STA interposition graft was made. A little back-flow from the M3 was detected when an incision was made in it. A second anastomosis between the M3 of the superior M2 trunk and the STA interposition graft was made. After performing the in situ M3-STA interposition graft-M3 bypass procedure, the inferior M2 trunk flow resumed, and the flattened wave on the SEP changed into a normal wave pattern (Fig. 2). The total time for the bypass procedure was 65 minutes. Immediate postoperative computed tomography (CT) angiography demonstrated good flow of the inferior M2 trunk with good patency of the bypass graft (Fig. 3). However, she was not awakened well, and additional CT on postoperative day one revealed some low density around the inferior M2 trunk territory. Emergency TFCA demonstrated occlusion of the inferior M2 trunk, and no visible bypass pedicle was detected (Fig. 3). At postoperative day three, she was awakened, but the motor power of her left upper arm was grade II. However, to our surprise, her left side weakness improved very rapidly, and nearly complete recovery was achieved at postoperative two weeks. Follow-up CT and TFCA at postoperative two weeks revealed much decreased low density in the M2 inferior trunk territory and complete recanalization of the M2 inferior trunk with an intact STA interposition graft (Fig. 4). The patient was discharged with no focal neurological deficits at postoperative three weeks.
Generally, in MCA aneurysm surgery clipping, aneurismal neck laceration is a well known complication, and its result usually has catastrophic cerebral ischemia in many cases1,3-5). So, bypass procedures have been reported to be helpful in clipping of complex middle cerebral artery aneurysms2,8). In our case, an aneurismal neck laceration happened during the clipping procedure due to severe atherosclerotic change in the aneurysm, and emergency suture of aneurismal neck was performed. However, flow resumption of the M2 inferior trunk was not achieved. Finally, we performed an M3-STA interposition graft-M3 anastomosis, and the flow of the M2 inferior trunk and MEP/SSEP were recovered.
Intracranial-intracranial (IC-IC) bypass is known as third-generation bypass surgery7) and has some benefits over extracranial-intracranial (EC-IC) bypass surgery, such as similar caliber of donor and recipient arteries, no need to harvest EC donor vessels, and less vulnerability to neck torsion or trauma6,7). However, in situ bypass between two MCA branches requires a more challenging side-to-side anastomosis between M3 with limited morbidity and has the co-sacrifice risk involving two MCA branches.
In this case, we used the frontal branch of the STA as an interposition graft because the parietal branch of the STA was sacrificed when the initial skin incision was made. The MEP change was recovered, and the normal wave patterns had been preserved until the end of surgery. Additionally, immediate postoperative CT angiography demonstrated good M2 trunk flow with good patency of the bypass graft. However, the patient was not easily roused, and acute cerebral infarction due to occlusion of the inferior M2 trunk developed. However, the occluded M2 inferior trunk was recanalized, and the low density at basal ganglia on CT disappeared almost at postoperative two weeks.
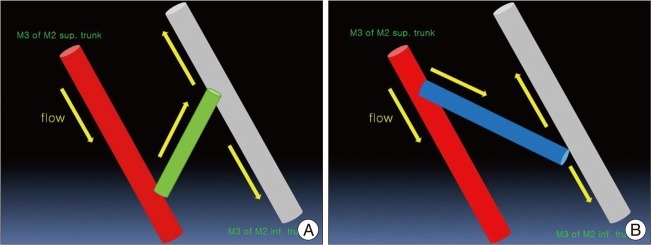
We suggest two important points. First, we think that the cause of delayed occlusion of the M2 inferior trunk after surgery is probably due to the direction of the free STA graft. This is demonstrated in the mimetic diagram (Fig. 5). Namely, the direction of flow is M3 of superior M2 trunk-STA interposition graft-M3 of inferior M2 trunk, but we performed an anastomosis with an acute angle between the M3 of superior M2 trunk and the STA graft with an obtuse angle between the STA graft and the M3 of M2 inferior trunk. Thus, the flow direction is not "natural", and the flow burden of the M2 superior trunk might have increased. If in this situation a small M2 inferior trunk flow around the MCA bifurcation resumed, flow "collision" might have occurred, and transient occlusion or low flow would have been sustained, leading to cerebral infarction. Second, we think that the small amount of flow through the M3-STA graft-M3 might have been sustained, and delayed spontaneous thrombolysis might have occurred. Finally, the origin of the MCA inferior trunk around the MCA bifurcation might have been open.
In any event, we obtained a final good result in this case with an emergency IC-IC bypass method; thus, if a STA-MCA anastomosis is not available under MCA flow obstruction, we can consider an emergency in situ MCA-MCA bypass procedure with or without an STA interposition graft. In addition, we must consider the "anastomosis angle" between the parent artery and free graft to get the natural flow direction.
We report an emergency in situ M3-M3 bypass procedure using an STA interposition graft as a rescue procedure in the case of an M2 trunk flow obstruction during clipping of an MCA aneurysm. If an STA-MCA anastomosis is not available under MCA flow obstruction, we can consider an emergency in situ MCA-MCA bypass procedure with or without an STA interposition graft.
Acknowledgements
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A102065).
References
1. Ahn JS, Kwun BD. Complications in middle cerebral artery aneurysm surgery. J Korean Neurosurg Soc. 1998; 27:1762–1768.
2. Bederson JB, Spetzler RF. Anastomosis of the anterior temporal artery to a secondary trunk of the middle cerebral artery for treatment of a giant M1 segment aneurysm. Case report. J Neurosurg. 1992; 76:863–866. PMID: 1564547.

3. Dashti R, Hernesniemi J, Niemelä M, Rinne J, Porras M, Lehecka M, et al. Microneurosurgical management of middle cerebral artery bifurcation aneurysms. Surg Neurol. 2007; 67:441–456. PMID: 17445599.

4. Karhunen PJ. Neurosurgical vascular complications associated with aneurysm clips evaluated by postmortem angiography. Forensic Sci Int. 1991; 51:13–22. PMID: 1752589.

5. Lanzino G, Spetzler RF. Clip wrapping for partial avulsion of the aneurysm neck. Technical note. J Neurosurg. 2003; 99:931–932. PMID: 14609177.
6. Quiñones-Hinojosa A, Lawton MT. In situ bypass in the management of complex intracranial aneurysms: technique application in 13 patients. Neurosurgery. 2005; 57:140–145. PMID: 15987580.

7. Sanai N, Zador Z, Lawton MT. Bypass surgery for complex brain aneurysms: an assessment of intracranial-intracranial bypass. Neurosurgery. 2009; 65:670–683. discussion 683. PMID: 19834371.
8. Seo BR, Kim TS, Joo SP, Lee JM, Jang JW, Lee JK, et al. Surgical strategies using cerebral revascularization in complex middle cerebral artery aneurysms. Clin Neurol Neurosurg. 2009; 111:670–675. PMID: 19595503.

Fig. 1
The sizes of the aneurysms were measured by transfemoral carotid angiography. A and B: Middle cerebral artery bifurcation aneurysm (11.3×9.0 mm). C: A2-3 aneurysm (7.1×3.1 mm).
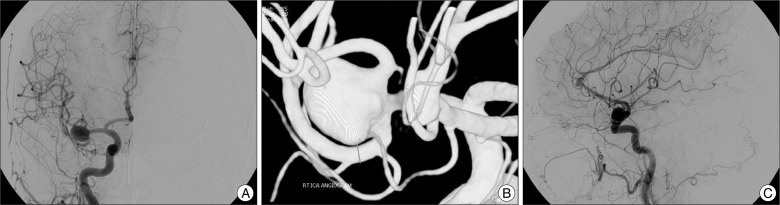
Fig. 2
Intraoperative evoked potential changes. After re-clipping of the middle cerebral artery aneurysm, SSEP (A) show a decreased amplitude near a flat wave (duration=55 minutes). After the M2-STA-M2 bypass procedure, recovered wave patterns are found in SSEP (B). SSEP: somatosensory evoked potential, STA: superficial temporal artery.
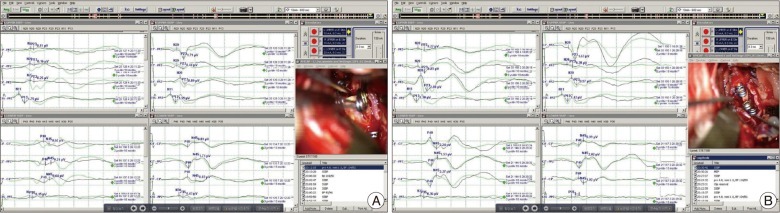
Fig. 3
A: Immediate postoperative computed tomography angiography demonstrates good M2 trunk flow of with good patency of the bypass graft. B: Emergency TFCA demonstrates occlusion of the inferior M2 trunk, and no visible bypass pedicle is detected. TFCA: transfemoral carotid angiography.
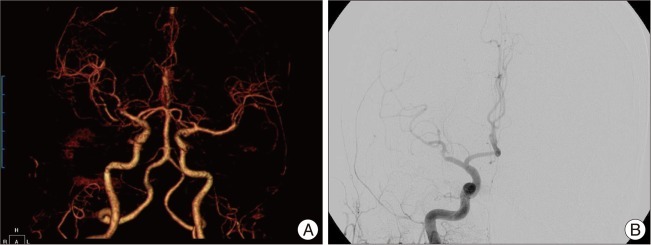
Fig. 4
Follow-up TFCA at postoperative two weeks reveals complete recanalization of the M2 inferior trunk (A) with intact STA interposition graft (B, white arrow). STA: superficial temporal artery, TFCA: transfemoral carotid angiography.
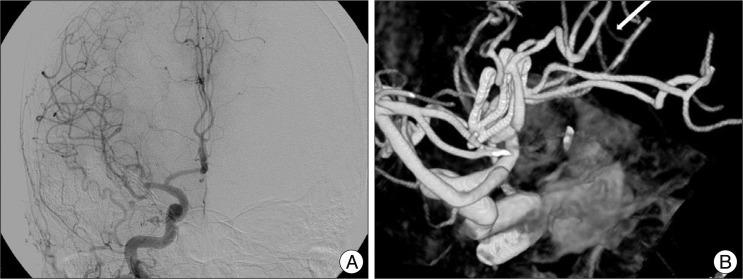
Fig. 5
The mimetic diagram of M3-STA-M3 anastomosis. A: In this case, we performed an anastomosis with an acute angle between the M3 of superior M2 trunk and the STA graft with an obtuse angle between the STA graft and the M3 of M2 inferior trunk. So, the flow direction is not "natural," and the flow burden of the M2 superior trunk might have increased. B: We believe that ideal and natural M3-STA-M3 anastomosis should be as diagrammed, with the natural flow direction from the M3 of superior M2 trunk toward the STA interposition graft with an obtuse angle. STA: superficial temporal artery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download