1. Aminoff MJ, Goodin DS, Barbaro NM, Weinstein PR, Rosenblum ML. Dermatomal somatosensory evoked potentials in unilateral lumbosacral radiculopathy. Ann Neurol. 1985; 17:171–176. PMID:
2983601.

2. Burke D, Skuse NF, Lethlean AK. Cutaneous and muscle afferent components of the cerebral potential evoked by electrical stimulation of human peripheral nerves. Electroencephalogr Clin Neurophysiol. 1981; 51:579–588. PMID:
6165559.

3. Date ES, Ortega HR, Hall K, Rappaport M. Somatosensory evoked responses to dermatomal stimulation in cervical spinal cord injured and normal subjects. Clin Electroencephalogr. 1988; 19:144–154. PMID:
3416499.

4. Dawson GD. Cerebral response to electrical stimulation of peripheral nerve in man. J Neurol Neurosurg Psychiatry. 1947; 10:134–140. PMID:
21610884.
5. Deletis V, Srala F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery : a review focus on the corticospinal tracts. Clin Neurophysiol. 2008; 119:248–264. PMID:
18053764.

6. Han TR, Kim JH, Kim HS. The significance of somatosensory evoked potential test in peripheral nerve injury. J Korean Acad Rehabil Med. 1991; 15:287–294.
7. Katifi HA, Sedgwick EM. Somatosensory evoked potentials from posterior tibial nerve and lumbo-sacral dermatomes. Electroencephalogr Clin Neurophysiol. 1986; 65:249–259. PMID:
2424736.

8. Kwon HK, Hwang MR, Yoon DW. Frequency and severity of carpal tunnel syndrome according to level of cervical radiculopathy : double crush syndrome? Clin Neurophysiol. 2006; 117:1256–1259. PMID:
16600675.

9. Napadow V, Kettner N, Ryan A, Kwong KK, Audette J, Hui KK. Somatosensory cortical plasticity in carpal tunnel syndrome--a cross-sectional fMRI evaluation. Neuroimage. 2006; 31:520–530. PMID:
16460960.

10. Owen JH, Padberg AM, Spahr-Holland L, Bridwell KH, Keppler L, Steffee AD. Clinical correlation between degenerative spine disease and dermatomal somatosensory-evoked potentials in humans. Spine (Phila Pa 1976). 1991; 16:S201–S205. PMID:
1862415.

11. Ra UW, Moon JH, Park ES, Cho KJ. Diagnosis of sensory radiculopathy after laminectomy. J Korean Acad Rehabil Med. 1984; 8:26–33.
12. Rodriquez AA, Kanis L, Rodriquez AA, Lane D. Somatosensory evoked potentials from dermatomal stimulation as an indicator of L5 and S1 radiculopathy. Arch Phys Med Rehabil. 1987; 68:366–368. PMID:
3036037.
13. Seo JH, Kim JY, Ko MH, Eun JP. Diagnostic usefulness of dermatomal somatosensory evoked potentials by low intensity stimulation in lumbar radiculopathy. J Korean Acad Rehabil Med. 2007; 31:341–345.
14. Stevens JC. American Association of Electrodiagnostic Medicine. AAEM minimonograph #26 : the electrodiagnosis of carpal tunnel syndrome. Muscle Nerve. 1997; 20:1477–1486. PMID:
9390659.

15. Sunderland S. Anatomical features of nerve trunks in relation to nerve injury and nerve repair. Clin Neurosurg. 1970; 17:38–62. PMID:
4939493.

16. Sunderland S. The nerve lesion in the carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 1976; 39:615–626. PMID:
993794.

17. Tinazzi M, Zanette G, Volpato D, Testoni R, Bonato C, Manganotti P, et al. Neurophysiological evidence of neuroplasticity at multiple levels of the somatosensory system in patients with carpal tunnel syndrome. Brain. 1998; 121:1785–1794. PMID:
9762965.

18. Tsai TM, Tsai CL, Lin TS, Lin CC, Jou IM. Value of dermatomal somatosensory evoked potentials in detecting acute nerve root injury: an experimental study with special emphasis on stimulus intensity. Spine (Phila Pa 1976). 2005; 30:E540–E546. PMID:
16166882.
19. Yazicioğlu K, Ozgül A, Kalyon TA, Gündüz S, Arpacioğlu O, Bilgiç F. The diagnostic value of dermatomal somatosensory evoked potentials in lumbosacral disc herniations : a critical approach. Electromyogr Clin Neurophysiol. 1999; 39:175–181. PMID:
10228885.
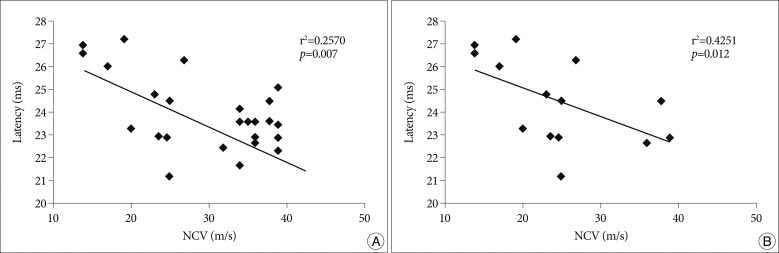
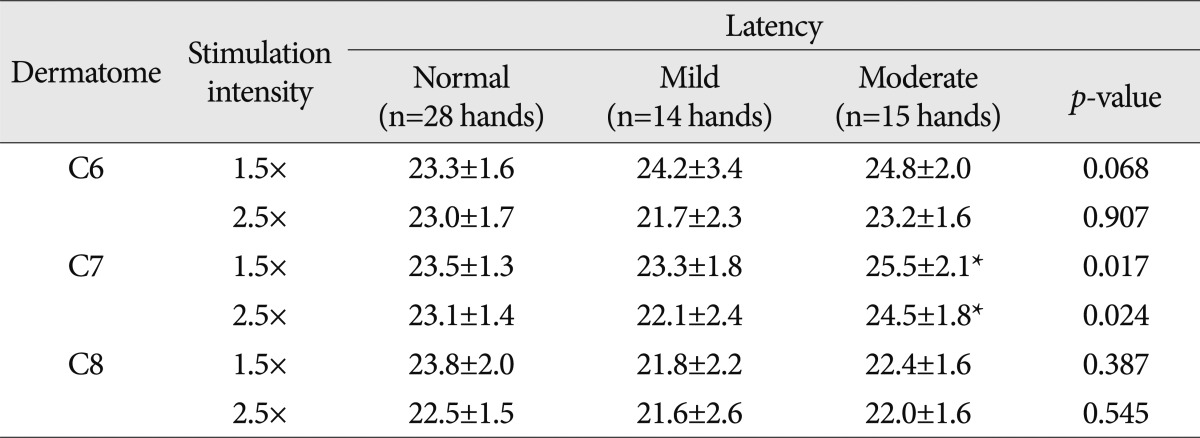
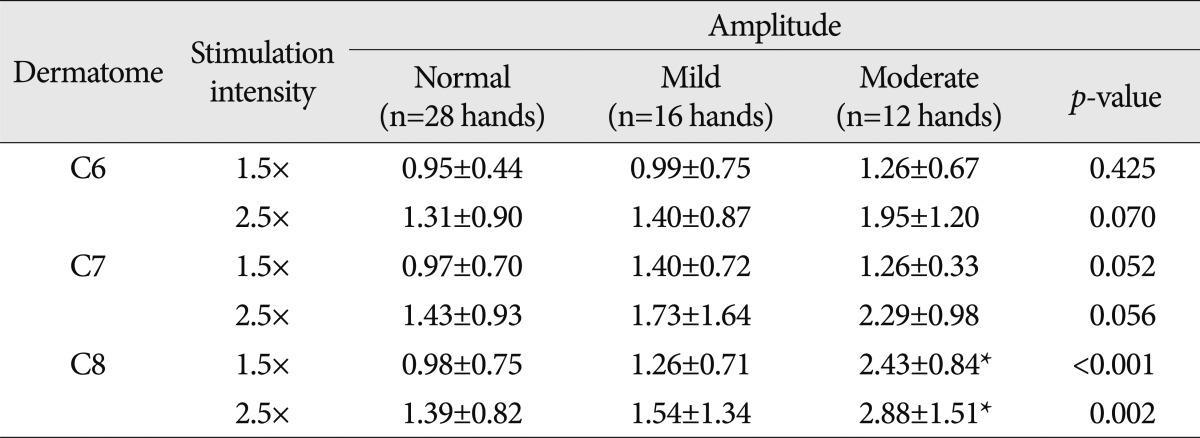




 PDF
PDF ePub
ePub Citation
Citation Print
Print


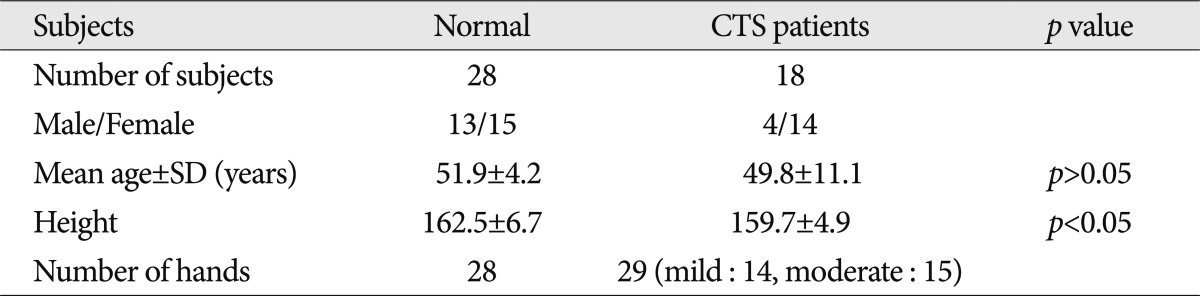
 XML Download
XML Download