Abstract
Parkinsonism secondary to intracranial mass lesions usually results from compression or distortion of the basal ganglia. Secondary parkinsonism due to midbrain infiltration or compression is rare and generally associated with other neurologic signs caused by pyramidal tract and/or cranial nerve involvement. We report a case of 30-year-old woman in whom mild parkinsonism was the major clinical manifestation of an astrocytoma in the anterior third ventricle and hypothalamus. She underwent surgical resection, ventriculoperitoneal shunt and radiation therapy. All symptoms of parkinsonism were completely recovered 3 months after the treatment. Brain tumors can be manifested only by the symptoms of parkinsonism. This case emphasizes the significance of neuroimaging in the evaluation of parkinsonism.
Secondary parkinsonism can be induced by drugs or various diseases, and its treatment and prognosis vary according to etiologic factors. Intracranial tumors rarely cause secondary parkinsonism. The majority of tumors causing secondary parkinsonism are extra-axial tumors which compress the basal ganglia; meningioma is the most common5,8). However, intra-axial tumors involving the basal ganglia or midbrain have rarely been reported in the literature8).
Since the first report of tuberculosis in the brainstem, there have been a few reported cases of parkinsonism secondary to space-occupying lesions compressing the substantia nigra1-4,10). However, in these cases, parkinsonism was usually associated with other neurologic signs and was not the main neurologic manifestation. There have been few reports of parkinsonism secondary to intracranial mass lesions without other neurological deficits2,4).
We report a first case of astrocytoma in the third ventricle and hypothalamus that presented with mild parkinsonism and no other neurological symptoms. A brief review of the literature has been included.
A 30-year-old woman visited our clinic with a 4-year history of tremor in both hands and a 6-month history of gait disturbance. The patient reported that the tremor in both hands and gait disturbance were progressively aggravated. On neurological examination, postural tremor was noted in both hands, but rest tremor was not observed. Rigidity was present in the extremities but not in the neck. On walking, arm swing was decreased. Mild bradykinesia was observed while the fingers were tapped, hands were closed and opened, and when foots were stomped on the floor. Facial expression was normal. Although postural tremor and rigidity were similar between both hands, brandykinesia was more severe in the left hand and arm swing on walking was more decreased in the left side. The patient showed independent walking with minor gait impairment and mild postural instability on the pull test; she recovered with 5 steps backwards for recovery without help. She complained of orthostatic dizziness but did not have orthostatic hypotension. Her consciousness was clear and orientation was well maintained. She revealed normal findings in the motor and sensory test, neurological tests for cerebellar function and eyeball movements, and deep tendon reflexes.
There was neither a notable history of medical disease nor a history of drug ingestion causing bradykinesia. Routine physical examinations including fundoscopy showed no abnormal findings. Nor were abnormal findings in the routine blood test, biochemical test, electrolyte test, thyroid function test, syphilis test, human immunodeficiency virus test, serum vitamins, serum cupper, serum ceruloplasmin, chest X-rays and electrocardiograms.
The patient was diagnosed with parkinsonism based on bradykinesia of both hands, rigidity and postural instability. Her score on the Unified Parkinson Disease Rating Scale was 22 points. Magnetic resonance (MR) images showed an ill-defined mass in the anterior third ventricle and hypothalamus, which had partially enhanced nodular intensity after gadolinium administration. The lesion was accompanied by hydrocephalus, and the midbrain was compressed and inferiorly displaced by the mass and obstructive hydrocephalus secondary to the tumor (Fig. 1). Based on these findings, the mass was suspected to be intraventricular diffuse astrocytoma.
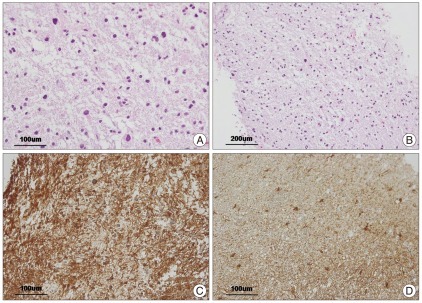
Under general anesthesia, a neuroendoscope was inserted into the third ventricle via a right frontal burr hole in February 2010. The neuronavigation system was used for planning the procedure. The lesion was visually confirmed with the neuroendoscope, therefore, partial surgical resection for biopsies was performed safely and precisely, and a right frontal external ventricular drain (EVD) was placed. Pathologic findings revealed diffuse astrocytoma grade II (Fig. 2). The postoperative computed tomography (CT) did not show any additional changes compared with preoperative findings. The postoperative course was uneventful without any neurological deficit.
However, one week later, she presented with mild deterioration of mental acuity after the EVD was removed. Follow-up brain CT did not show any changes compared with previous findings. The patient underwent neuronavigation guided endoscopic septostomy and ventriculoperitoneal (VP) shunt which is performed subsequently and independently after partial surgical resection in February 2010. A Hakim VP shunt with a high pressure valve was placed through another bur hole in the left frontal region (Codman, Raynham, MA, USA). The preoperative hydrocephalus was normalized after VP shunt. The patient then received radical high dose fractionated three-dimensional conformal radiation therapy with an overall dose of 54 Gy from March 2010 to April 2010, followed by being treated with steroid medication. Consequently, the tumor and hydrocephalus were significantly decreased (Fig. 1). All symptoms of parkinsonism, including tremor, bradykinesia, rigidity and gait disturbance, were completely recovered 3 months after treatment.
Parkinsonism can occur due to intracranial tumors at various sites, but it usually develops by compression of the basal ganglia by supratentorial tumors3,5,8). There have been only a few reported cases of parkinsonism due to space-occupying lesions compressing the substantia nigra of the midbrain in the literature1-4,10).
The pathophysiology of parkinsonism due to brain tumors has not yet been elucidated. It has been reported that parkinsonism can be induced by direct compression of the basal ganglia and nigrostriatal structures such as the midbrain by tumors, indirect compression by a herniated temporal lobe and secondary displacement of the brainstem by tumors3,10). Several reports have confirmed that in patients with parkinsonism induced through compression of nigrostriatal structures by brain tumors, clinical symptoms are improved after removing the tumors7,8,10). Similarly, in our patient who showed midbrain compression in MR imaging, clinical symptoms were improved after tumor reduction. Based on these results, it is conceivable that compression of nigrostriatal structures in the brainstem by brain tumors can lead to parkinsonism.
In our patient, MR images also revealed the findings of hydrocephalus. Hydrocephalus is known to induce parkinsonism. Patients with parkinsonism induced by hydrocephalus usually show wide-based gait, well maintained arm shaking on walking and clinical symptoms and signs such as signs of increased intracranial pressure, recognition deficits including memory function and urinary incontinence9). However, our patient showed normal steps, decreases of arm shaking on walking and none of the aforementioned clinical symptoms and signs. It may inferred that hydrocephalus did not significantly contribute to the development of parkinsonism in our case. But, clinical symptoms improved after reduction of hydrocephalus by cerebrospinal fluid drainage, we therefore suspected midbrain compression by obstructive hydrocephalus secondary to the tumor as another possible cause of parkinsonism.
Parkinsonism secondary to intracranial tumors usually belongs to akinetic-rigid syndrome that usually does not respond to levodopa and is associated with unilateral manifestation along with neurological deficit such as impairment of eyeball movement, pyramidal signs and cerebellar dysfunction6,8). However, in our patient, tremor and gait disturbance were the main symptoms. In addition, pure bilateral parkinsonism without any other neurological abnormalities was also noted. These findings are different from those of secondary parkinsonism. However, our patient seemed to have secondary parkinsonism because : 1) the patient's age was not the usual for primary parkinsonism, 2) the clinical course progressed more rapidly and postural instability occurred earlier as compared to early onset parkinson's disease.
Although parkinsonism secondary to brain tumors is relatively rare, brain tumors can be manifested only by the symptoms of parkinsonism as in our case. In such cases, if secondary parkinsonism is not suspected, its diagnosis may be delayed and can cause more severe permanent neurological deficits. Although a fundamental treatment method for most cases of secondary parkinsonism has not yet been established, parkinsonism secondary to brain tumors can be completely cured with early diagnosis and appropriate treatment. Therefore, brain imaging studies are necessary to detect accurate causes of secondary parkinsonism when patients show a different onset age or disease progression from primary parkinsonism as in our patient, as well as when they show neurological abnormalities other than symptoms of parkinsonism or do not respond to levodopa with unilateral manifestation.
References
1. Blocq P, Marinesco G. [Sur un cas tremblement Parkinsonien hémiplégique symptomatique d, une tumeur de pédoncule cérébral]. CR Cos Biol (Paris). 1893; 45:105–111.
2. Cicarelli G, Pellecchia MT, Maiuri F, Barone P. Brain stem cystic astrocytoma presenting with "pure" parkinsonism. Mov Disord. 1999; 14:364–366. PMID: 10091637.

3. Gherardi R, Roualdes B, Fleury J, Prost C, Poirier J, Degos JD. Parkinsonian syndrome and central nervous system lymphoma involving the substantia nigra. A case report. Acta Neuropathol. 1985; 65:338–343. PMID: 3872004.

4. Gouider-Khouja N, Gabsi S, Khouja N, Hentati F. Hemiparkinsonian syndrome due to a cerebral tumor infiltrating the substantia nigra. Parkinsonism Relat Disord. 2000; 6:115–117. PMID: 10699394.

5. Husag L, Wieser HG, Probst C. [Extrapyramidal symptoms in meningiomas]. Schweiz Arch Neurol Neurochir Psychiatr. 1975; 116:257–279. PMID: 1153969.
6. Krauss JK, Paduch T, Mundinger F, Seeger W. Parkinsonism and rest tremor secondary to supratentorial tumours sparing the basal ganglia. Acta Neurochir (Wien). 1995; 133:22–29. PMID: 8561031.

7. Leenders KL, Findley LJ, Cleeves L. PET before and after surgery for tumor-induced parkinsonism. Neurology. 1986; 36:1074–1078. PMID: 3488518.

8. Polyzoidis KS, McQueen JD, Rajput AH, MacFadyen DJ. Parkinsonism as a manifestation of brain tumor. Surg Neurol. 1985; 23:59–63. PMID: 2981121.

9. Sypert GW, Leffman H, Ojemann GA. Occult normal pressure hydrocephalus manifested by parkinsonism-dementia complex. Neurology. 1973; 23:234–238. PMID: 4735176.

10. Wakai S, Nakamura K, Niizaki K, Nagai M, Nishizawa T, Yokoyama S, et al. Meningioma of the anterior third ventricle presenting with parkinsonism. Surg Neurol. 1984; 21:88–92. PMID: 6689817.

Fig. 1
Brain magnetic resonance imaging show diffuse astrocytoma in the anterior third ventricle and hypothalamus. Sagittal FLAIR image (A), T2-weighted axial image (B), and T1-weighted axial image after administration of gadolinium (C) show an ill-defined lesion with nodular enhancement, which involves the anterior third ventricle and bilateral hypothalamis (arrowhead). The images before treatment also show compression and inferiorly displacement of the midbrain including the substantia nigra by the tumor mass and obstructive hydrocephalus secondary to the tumor (arrow). The images after treatment with surgical excision and cerebrospinal fluid drainage show significant decrease of the tumor and lateral ventricles, and consequent improvement of midbrain compression (D, E and F).
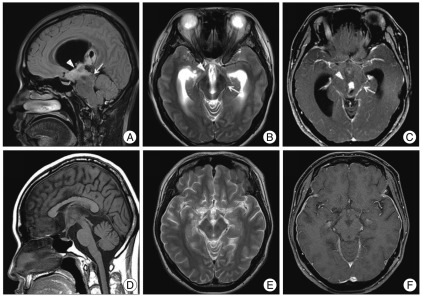
Fig. 2
These photomicrographs are histopathologic findings of diffuse astrocytoma (WHO grade II). Higher magnification of the tumor shows a hypercellular lesion composed of cells with moderate plemorphism and chromatin density (A : H&E stain, ×200; B : H&E stain, ×100). Immunohistochemistry reveals diffuse positivity for GFAP in the tumor cells (C : avidin-biotin-peroxidase, ×200) compare with in the gliosis (D : avidin-biotin-peroxidase, ×200).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download