Abstract
Objective
Placement of a single transverse stent via the nondominant A1 across the anterior communicating artery (AComA) into the contralateral A2 can provide sufficient neck coverage for wide-necked bifurcation AComA aneurysms. The authors described the feasibility, safety and long-term outcomes of this technique.
Methods
Between January 2015 and February 2018, placement of a single transverse stent via the nondominant A1 was attempted in 17 wide-necked bifurcation AComA aneurysms. The authors reviewed the medical records and radiological studies.
Results
The technical success rate was 94.1% (16/17). Periprocedural thromboembolic complications occurred in one patient (6.3%) without permanent neurological deficits. The mean clinical follow-up duration was 39.9±9.8 months. No deaths or delayed thromboembolic complications occurred. The mean angiographic follow-up duration was 38.9±9.8 months. The immediate and final follow-up complete occlusion rates were 87.4 and 93.7%, respectively. There was no recanalization during the follow-up period.
Conclusion
Placement of a single transverse stent via the nondominant A1 across the AComA into the contralateral A2 is a feasible and relatively safe endovascular technique for the treatment of wide-necked bifurcation AComA aneurysms, with good long-term occlusion rates and a reasonable complication rate, if only the nondominant A1 is applicable.
The anterior communicating artery (AComA) is the most common site of aneurysmal rupture [21]. Given the high risk of rupture of AComA aneurysms, it is crucial to properly treat aneurysms at this site. In general, due to hemodynamic influence, many AComA aneurysms arise from the dominant anterior cerebral artery (ACA) and are formed like bifurcation aneurysms among the dominant A1, AComA and ipsilateral A2 [17,25]. For wide-necked aneurysms, sometimes, single stenting from A1 to A2 is not sufficient to cover the neck to protect coils from protruding into the AComA. To overcome this limitation, several techniques using double stents, such as X, Y, and T stenting, have been reported [6,8,9,14,19]. However, double stenting has several disadvantages. A crossing point occurs between the two stents, which may result in a risk of acute or chronic thromboembolism due to insufficient wall apposition and endothelialization. In our institution, to overcome these disadvantages, placement of a single transverse stent via the nondominant A1 across the AComA into the contralateral A2 was selectively performed in coil embolization of wide-necked bifurcation AComA aneurysms. The aneurysms were coiled via the opposite (dominant) A1. To the best of our knowledge, many previous studies on endovascular treatment of wide-necked AComA aneurysms have not addressed this technique [3-5,7,8,10-12,18,22]. Herein, we described the feasibility, safety and long-term outcomes of this technique.
The Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-2012/652-106) approved this study, and informed consent was waived because the data and images could not be identified with the individual patients.
Between January 2015 and February 2018, 238 AComA aneurysms were treated with coil embolization, including 74 stent-assisted cases. Of the 74 stent-assisted coiling (SAC) cases, placement of a single transverse stent via the nondominant A1 through the AComA was attempted in 17 wide-necked bifurcation AComA aneurysms under the following conditions : 1) presence of a nondominant A1 and AComA that could be accessed with a microcatheter; 2) technical feasibility of navigation across an acute angle between the nondominant A1 and the AComA using a microcatheter; and 3) difficulty in obtaining sufficient aneurysm neck coverage with other single-stent placement techniques, such as the dominant A1 to ipsilateral A2 or contralateral A2. We retrospectively reviewed patients’ medical records and pre- and posttreatment radiological images. A wide-necked AComA aneurysm was defined as an aneurysm with a neck diameter of >4.0 mm or an aspect ratio of <1.5. A dominant A1 was defined as a large A1, that had a diameter twice that of the small A1 segment (nondominant A1). Angioarchitectural features of the AComA complex including aneurysm size, diameters of parent arteries and number of AComA perforators, were analyzed using three-dimensional (3D) imaging with VitreaWorkstationTM (Vital Images Incorporated, Minnetonka, MN, USA). Consensus on the results was reached by two experienced endovascular neurosurgeons (S.P.B. and Y.D.K.).
All enrolled patients received 100 mg of aspirin and 75 mg of clopidogrel for at least 5 days before embolization. One day prior to coil embolization, P2Y12 reaction units (PRU) were measured using VerifyNow (Accumetrics, San Diego, CA, USA). Patients with poor clopidogrel response (greater than 220 PRU) were switched to prasugrel, with a loading dosage of 20 mg followed by a maintenance dosage of 5 mg. All endovascular procedures were performed under general anesthesia. A 7-French (Fr) introducer sheath was inserted into the right femoral artery, and another 6-Fr introducer sheath was inserted into the left femoral artery. A 3000 IU bolus dose of intravenous heparin was administered after the placement of the femoral introducer sheath, and heparin was later infused at an hourly booster dose of 1000 IU with monitoring of the activated clotting time. A 7-Fr guiding catheter (Envoy; Codman Neurovascular, Raynham, MA, USA) was placed at the proximal cervical internal carotid artery (ICA) on the dominant A1 side via a 7-Fr sheath. Another 6-Fr Envoy guiding catheter was placed at the proximal cervical ICA on the nondominant A1 side via a 6-Fr sheath. The AComA complex, including the aneurysm, was well visualized only through ICA angiography on the dominant A1 side during the procedure, so a microcatheter (Excelsior SL-10; Stryker Neurovascular, Kalamazoo, MI, USA) for coil delivery was jailed into the aneurysm sac through the dominant A1 before stent deployment. Another Excelsior SL-10 microcatheter only for stent delivery was placed via the nondominant A1 across the AComA and A2 on the dominant A1 side. Selection of stent size mainly depends on the diameter of the parent arteries. The smallest 2.5-mm Low-profile Visualized Intraluminal Support Junior stent (LVIS Jr; MicroVention, Tustin, CA, USA) in various length was used for all cases because the diameter of the parent arteries, with a deployed stent, was smaller than 2.5 mm. A LVIS Jr stent was intentionally deployed across the aneurysm neck so that the proximal tip of the stent could end at the junction of nondominant A1 and AComA. Then, the aneurysm was packed using bare platinum coils until complete occlusion was achieved or further coiling was deemed unsafe (Fig. 1). Finally, after confirming the patency of the stent and parent arteries in digital subtraction angiography (DSA), the procedure was terminated. Both femoral puncture sites were sealed using closure devices (Perclose ProGlide; Abbott, Santa Clara, CA, USA). After the procedure, dual antiplatelet treatment continued for 1 year; after 1 year, this therapy was exchanged for daily oral treatment with 100 mg of aspirin.
The clinical outcomes of all patients were evaluated using a modified Rankin Scale (mRS) score on admission and at the last outpatient follow-up visit. Intraprocedural rupture and thromboembolic events were evaluated. Newly developed neurological deficits associated with any hemorrhage or ischemic lesions during follow-up periods were also reviewed.
According to our institutional protocol, patients underwent imaging follow-up at 3 and 6 months with skull plain radiography and at 1, 2, 3, and 5 years with 3D time-of-flight magnetic resonance angiography (MRA) including source imaging. Any time a major recanalization was found, DSA was performed. DSA follow-up studies were routinely performed between 3 and 5 years after coil embolization if no major recanalization of an aneurysm was suspected. The patency of parent arteries and AComA perforators was documented by follow-up DSA. If follow-up DSA was not performed, vessel patency was evaluated using the follow-up axial MRA source image with identifying the new cerebral infarction in the AComA perforator territory. Aneurysm obliteration grades were classified as described by the Roy-Raymond occlusion classification : complete, residual neck, or residual aneurysm [23] and recanalization was defined as deterioration in the Roy-Raymond occlusion classification.
The baseline characteristics of the treated patients and aneurysms are summarized in Table 1. Ten patients (62.5%) were female and the mean age was 64.1±8.3 years (range, 44.0–78.0). Thirteen aneurysms that had not received prior treatment were unruptured, and three were recurrent with a history of previous treatment by coil embolization for subarachnoid hemorrhage (SAH). The mean maximum aneurysm diameter was 5.03±1.62 mm (range, 3.10–8.40), and the mean neck size was 3.89±0.89 mm (range, 3.00–6.60). The mean aspect ratio was 0.88±0.16 (range, 0.64–1.10). Twelve (75.0%) of the 16 patients had dominant A1 on the left. The mean diameter of arteries was 2.39±0.19 (range, 2.03–2.68) for dominant A1, 1.27±0.25 mm (range, 0.91–1.89) for AComA, and 1.10±0.12 mm (range, 0.86–1.28) for nondominant A1. The mean number of AComA perforators was 1.3±0.6 (range, 0–2).
Of the 17 attempted cases, technical success was achieved in 16 cases (94.1%) (Fig. 2). In one patient, navigation of the AComA with a microcatheter failed due to severe tortuosity between the nondominant A1 and AComA.
The outcomes of patients are summarized in Table 2. The mean clinical follow-up duration was 39.9±9.8 months (range, 22–51). The preoperative mRS score was 0 in 13 patients (81.3%) and 1 in three patients (18.7%) because of a previous history of SAH in two patients and cerebral infarction in one patient. The final follow-up mRS score was unchanged. Periprocedural thromboembolic complications occurred in one patient (6.3%). There were no intraprocedural complications or immediate neurological deficits, but 6 hours after the procedure, the patient presented right side weakness (strength of 2/5). There were multiple scattered infarctions in the ACA-middle cerebral artery border zone area on MR images, and left small ACA cortical branch occlusion was detected without in-stent thrombus formation on DSA. Intraarterial bolus infusion of tirofiban through a microcatheter was performed. Because stent-related microemboli were thought to be the cause of infarction, although the clopidogrel response was within the normal range (176 PRU) in this patient, clopidogrel was switched to prasugrel. The patient’s mRS score at discharge was zero after conservative and rehabilitation treatment (Fig. 3). There were no deaths or delayed thromboembolic complications in this study.
The mean angiographic follow-up duration was 38.9±9.8 months (range, 22–51). Immediate postoperative Raymond-Roy occlusion classification class 1 was achieved in 14 patients (87.4%), class 2 in one patient (6.3%), and class 3 in one patient (6.3%). The final follow-up angiography (nine cases were measured by DSA and seven cases were measured by MRA with source imaging) revealed class 1 in 15 patients (93.7%) and class 2 in one patient (6.3%). There was no recanalization during the follow-up period. In seven cases of MRA follow-up, it was difficult to clearly confirm the patency of the AComA complex due to MR artifacts, but in nine cases of DSA follow-up, the AComA complex, with a deployed stent including AComA perforators, had persistent blood flow without severe luminal narrowing. In addition, there was no newly detected cerebral infarction during the follow-up period in any cases.
In coil embolization of wide-necked bifurcation AComA aneurysms, maintaining the patency of the surrounding vessels, including perforators, is crucial for successful endovascular treatment. Various treatment techniques have been developed, including using multiple stents and unique single stenting techniques, and instruments [2,4,8,9,11,13-16,19,20,22]. In this study, we presented a technique involving transverse stenting through the AComA via the nondominant A1.
AComA aneurysms usually occurred at the junction of the dominant A1 and AComA (81.3%). Only 18.7% of AComA aneurysms occurred in the middle of the AComA [24]. Therefore, when application of a stent is needed, it is often placed via the dominant A1 to the ipsilateral A2 [10]. Likewise, when placement of a stent is needed, it is also placed via the dominant A1 because the nondominant A1 is small and the AComA complex is well observed through ICA angiography on the dominant A1 side. Given this limitation, to treat wide-necked AComA aneurysms, stent placements were undertaken by five routes : 1) dominant A1 to ipsilateral A2; 2) dominant A1 to contralateral A2; 3) dominant A1 to contralateral A1; 4) dominant A1 to aneurysm (waffle-cone technique or new stents for bifurcation aneurysms such as the pCONus [phenox] and PulseRider [Pulsar Vascular]); and 5) dominant A1 to both A2 (double stenting) [2,4,8,9,11,13-16,19,20,22]. However, single stenting through the dominant A1 to the ipsilateral A2 or contralateral A2 may not provide sufficient neck coverage for wide-necked bifurcation AComA aneurysms, so coils can protrude. For this reason, to provide more sufficient neck coverage, methods for direct stenting into aneurysms, such as the waffle-cone technique, pCONus or PulseRider placements and double stenting technique, have been reported [2,6,8,9,14-16,19]. However, these methods also have several disadvantages. Methods for direct stenting into aneurysms have the potential to increase the risk of rupture during the procedure due to the sharp stent end being in the aneurysm and the increase in recurrence rate due to augmentation of direct blood flow to the aneurysm [2,15,16]. Double stenting techniques seem to increase the risk of acute or chronic thromboembolism due to insufficient wall apposition and endothelialization [4,5,7-9,12]. These factors may affect the poor initial or follow-up occlusion results and increase periprocedural complications [10,22].
To date, in performing SAC of wide-necked AComA aneurysms, the periprocedural complication rates were high with double stenting techniques, while the recanalization and retreatment rates were high with single stenting techniques [1,4,5,7-10,12,18,22]. Periprocedural complication rates associated with single SAC of wide-necked AComA aneurysms were 4.8–11.0% [1,10,18,22]. The complete occlusion rates were 43.2–44.0% and 72.7–86.9% at the initial and last times, respectively. The recanalization rate was 8.3–13.1%, and retreatment rate was 3.4–8.1%. On the other hand, according to several studies that have assessed double SAC, such as X, Y, and T stenting, the complete occlusion rate was 85.8–95.7%, with procedural complication rates of 6.7–17.5%4,5,7-9,12). The recanalization and retreatment rates were 0.0–7.8% and 0.0–2.4%, respectively. To avoid using double stents and risks associated with placing stents into aneurysms, we performed a new single transverse stenting technique via the nondominant A1 across the AComA and contralateral A2. This technique provides several advantages, including full aneurysm neck coverage across the AComA complex and a potential flow-diversion effect by dual layer stent strut protection from dominant A1 blood flow to aneurysm. In the present study, the complete occlusion rate was 93.7%, which was similarly high to the results of double stenting techniques, while the periprocedural complication rate of 6.3% was similarly low to the result of single stenting techniques. Although one patient experienced a periprocedural thromboembolic event, this event was more likely to have been a complication from the use of a stent than from this technique, and the patient fully recovered before discharge. Furthermore, during the follow-up periods, there were no recanalization events or delayed thromboembolic complications, including AComA perforator infarction. It is thought that this technique could reduce the recanalization rate by decreasing the hemodynamic stress to the aneurysm due to double-layer stent strut protection and decrease periprocedural thromboembolic complications by inducing endothelialization due to sufficient wall apposition. There could be concerns about patency of the AComA and small nondominant A1 with a stent. In our series, A1 and AComA patency was well maintained during the follow-up period. However, several anatomic conditions in the AComA complex are required to perform this stenting technique, such as the presence of both A1 and the size of the nondominant A1 and AComA, which should allow the passage of microcatheters and acceptable arterial tortuosity. We had one case in which crossing the AComA with a microcatheter failed due to severe tortuosity between the nondominant A1 and AComA. Femoral artery puncture can also be a concern. Although both femoral artery punctures and a dual guiding system were applied in this study because the jailing technique was preferred during SAC in our institution, there was no related complications. However, both femoral artery puncture and the dual guiding system may have additional risks of thromboembolic complications and puncture site problems. Single puncture and one guiding system might be possible. The aneurysm can be selected via dominant A1 after stent placement via nondominant A1 using a single guiding catheter.
Similar to our technique, dominant A1-AComA-contralateral A1 stenting to completely cover the aneurysmal neck only when the AComA aneurysm was in the middle of the AComA with symmetric A1 on both sides has been reported [22,24]. Therefore, this stenting technique is very rarely applicable and cannot provide sufficient neck coverage in wide-necked bifurcation AComA aneurysms.
Our study has severe limitations, including its retrospective nature and small sample size from a single institution. In seven cases, follow-up imaging was performed with MRA according to our institutional protocol. Although the patency of both the A1 and A2 was confirmed and there was no AComA perforator infarction on follow-up MR images, the patency of the AComA was not accurately confirmed due to the metal artifact of the stent. In cases of A1 aplasia or very small A1 that cannot be navigated with a microcatheter, this technique cannot be applied. Further prospective and large sample size studies are warranted to more completely understand the efficacy and safety of this technique.
Our study shows that placement of a single transverse stent via the nondominant A1 across the AComA into the contralateral A2 is a feasible and relatively safe endovascular technique for the treatment of wide-necked bifurcation AComA aneurysms with good long-term occlusion rates and a reasonable complication rate. This technique can be a good option for treating wide-necked bifurcation AComA aneurysms if only the nondominant A1 is applicable.
ACKNOWLEDGMENTS
This study is supported by a fund from Seoul National University Bundang Hospital (06-2013-087).
References
1. Adeeb N, Griessenauer CJ, Patel AS, Foreman PM, Baccin CE, Moore JM, et al. The use of single stent-assisted coiling in treatment of bifurcation aneurysms: a multicenter cohort study with proposal of a scoring system to predict complete occlusion. Neurosurgery. 82:710–718. 2018.

2. Aguilar-Pérez M, Kurre W, Fischer S, Bäzner H, Henkes H. Coil occlusion of wide-neck bifurcation aneurysms assisted by a novel intra- to extra-aneurysmatic neck-bridging device (pCONus): initial experience. AJNR Am J Neuroradiol. 35:965–971. 2014.

3. Ahmed ME, Lum C, Lesiuk H, Iancu D, dos Santos M. Navigation of stents across communicating arteries for aneurysm embolization. Can J Neurol Sci. 41:193–199. 2014.

4. Aydin K, Balci S, Sencer S, Barburoglu M, Umutlu MR, Arat A. Y-stent-assisted coiling with low-profile neuroform atlas stents for endovascular treatment of wide-necked complex intracranial bifurcation aneurysms. Neurosurgery. 87:744–753. 2020.

5. Aydin K, Men S, Barburoglu M, Sencer S, Akpek S. Initial and Long-term outcomes of complex bifurcation aneurysms treated by Y-stent-assisted coiling with low-profile braided stents. AJNR Am J Neuroradiol. 39:2284–2290. 2018.

6. Aydin K, Sencer S, Barburoglu M, Berdikhojayev M, Aras Y, Sencer A, et al. Midterm results of T-stent-assisted coiling of wide-necked and complex intracranial bifurcation aneurysms using low-profile stents. J Neurosurg. 127:1288–1296. 2017.

7. Aydin K, Stracke CP, Barburoglu M, Yamac E, Berdikhojayev M, Sencer S, et al. Long-term outcomes of wide-necked intracranial bifurcation aneurysms treated with T-stent-assisted coiling. J Neurosurg. 134:39–48. 2021.

8. Bartolini B, Blanc R, Pistocchi S, Redjem H, Piotin M. “Y” and “X” stent-assisted coiling of complex and wide-neck intracranial bifurcation aneurysms. AJNR Am J Neuroradiol. 35:2153–2158. 2014.

9. Cagnazzo F, Limbucci N, Nappini S, Renieri L, Rosi A, Laiso A, et al. Y-stent-assisted coiling of wide-neck bifurcation intracranial aneurysms: a meta-analysis. AJNR Am J Neuroradiol. 40:122–128. 2019.

10. Choi HH, Cho YD, Yoo DH, Ahn SJ, Cho WS, Kang HS, et al. Stentassisted coil embolization of anterior communicating artery aneurysms: safety, effectiveness, and risk factors for procedural complications or recanalization. J Neurointerv Surg. 11:49–56. 2019.

11. Choi HH, Cho YD, Yoo DH, Lee SH, Yeon EK, Kang HS, et al. Safety and efficacy of anterior communicating artery compromise during endovascular coil embolization of adjoining aneurysms. J Neurosurg. 132:1068–1076. 2019.

12. Ciccio G, Robert T, Smajda S, Fahed R, Desilles JP, Redjem H, et al. Double stent assisted coiling of intracranial bifurcation aneurysms in Y and X configurations with the Neuroform ATLAS stent: immediate and mid term angiographic and clinical follow-up. J Neurointerv Surg. 11:1239–1242. 2019.

13. Du EHY, Shankar JJS. LVIS Jr ‘shelf’ technique: an alternative to Y stentassisted aneurysm coiling. J Neurointerv Surg. 8:1256–1259. 2016.

14. Fargen KM, Mocco J, Neal D, Dewan MC, Reavey-Cantwell J, Woo HH, et al. A multicenter study of stent-assisted coiling of cerebral aneurysms with a Y configuration. Neurosurgery. 73:466–472. 2013.

15. Gory B, Spiotta AM, Mangiafico S, Consoli A, Biondi A, Pomero E, et al. PulseRider stent-assisted coiling of wide-neck bifurcation aneurysms: periprocedural results in an international series. AJNR Am J Neuroradiol. 37:130–135. 2016.

16. Guo XB, Yan BJ, Guan S. Waffle-cone technique using solitaire AB stent for endovascular treatment of complex and wide-necked bifurcation cerebral aneurysms. J Neuroimaging. 24:599–602. 2014.

17. Hassan T, Elsayed A, Abbas M, Sultan A, Eladawy Y. Proposed parent vessel geometry based classification of anterior communicating artery-located aneurysms. World Neurosurg. 101:259–269. 2017.

18. Huang Q, Xu Y, Hong B, Zhao R, Zhao W, Liu J. Stent-assisted embolization of wide-neck anterior communicating artery aneurysms: review of 21 consecutive cases. AJNR Am J Neuroradiol. 30:1502–1506. 2009.

19. Kwon HJ, Lim JW, Byoun HS, Koh HS. Novel noncrossing Y-stent technique using tapered proximal end of a solitaire AB stent for coil embolization of wide-neck bifurcation aneurysms. J Korean Neurosurg Soc. 64:136–141. 2021.

20. Lescher S, du Mesnil de Rochemont R, Berkefeld J. Woven Endobridge (WEB) device for endovascular treatment of complex unruptured aneurysms-a single center experience. Neuroradiology. 58:383–390. 2016.

21. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 360:1267–1274. 2002.

22. Raslan AM, Oztaskin M, Thompson EM, Dogan A, Petersen B, Nesbit G, et al. Neuroform stent-assisted embolization of incidental anterior communicating artery aneurysms: long-term clinical and angiographic follow-up. Neurosurgery. 69:27–37. discussion 37. 2011.

23. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 32:1998–2004. 2001.

24. Yasargil MG. Microsurgery II. Anterior Cerebral and Anterior Communicating Artery Aneurysms. Stuttgart: Geroge Thieme Verlag;1984.
Fig. 1.
Illustration of single transverse stenting via the nondominant A1 in coil embolization of an AComA aneurysm. A : The A2 segment on the dominant A1 side (arrow) is catheterized for stent delivery via the nondominant A1 (arrowhead) and AComA. Another microcatheter for coil delivery is located in the aneurysm through the dominant A1. B : A low-profile stent is deployed in order to fully cover the aneurysm neck. C : Coil embolization is performed under the protection of a deployed stent. AComA : anterior communicating artery.
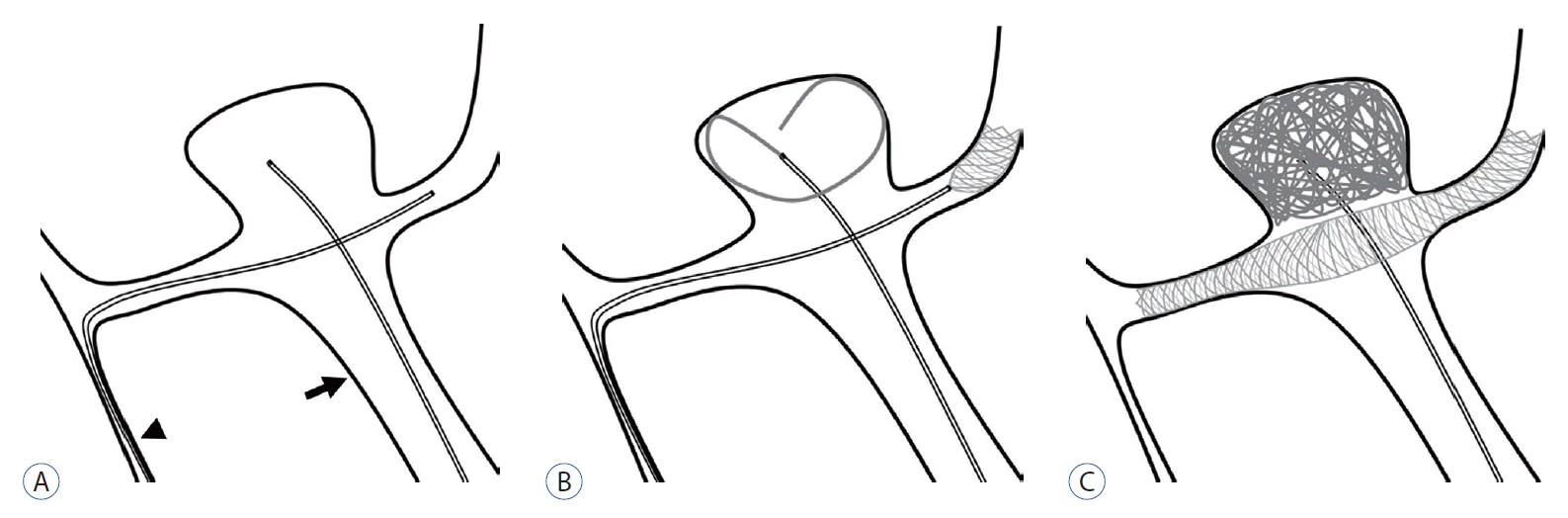
Fig. 2.
A wide-necked AComA aneurysm treated with single transverse stenting technique. A : DSA image showed a wide-necked bifurcation AComA aneurysm (arrow). B : After a microcatheter for coil delivery was navigated into the AComA aneurysm (dotted-line), right internal carotid artery angiography showed the nondominant A1 and AComA aneurysm in hazy form. C : A low-profile stent (LVIS Jr stent, 2.5×13 mm; MicroVention, Tustin, CA, USA) was inserted through a microcatheter that was navigated in the A2 on the dominant A1 side (arrowheads). D : The stent was deployed to fully cover the aneurysm neck. E : Bare platinum coils were packed until complete occlusion was achieved or further coiling was deemed unsafe. F : Immediate postembolization DSA showed that the aneurysm was completely occluded. DSA : digital subtraction angiography, AComA : anterior communicating artery.
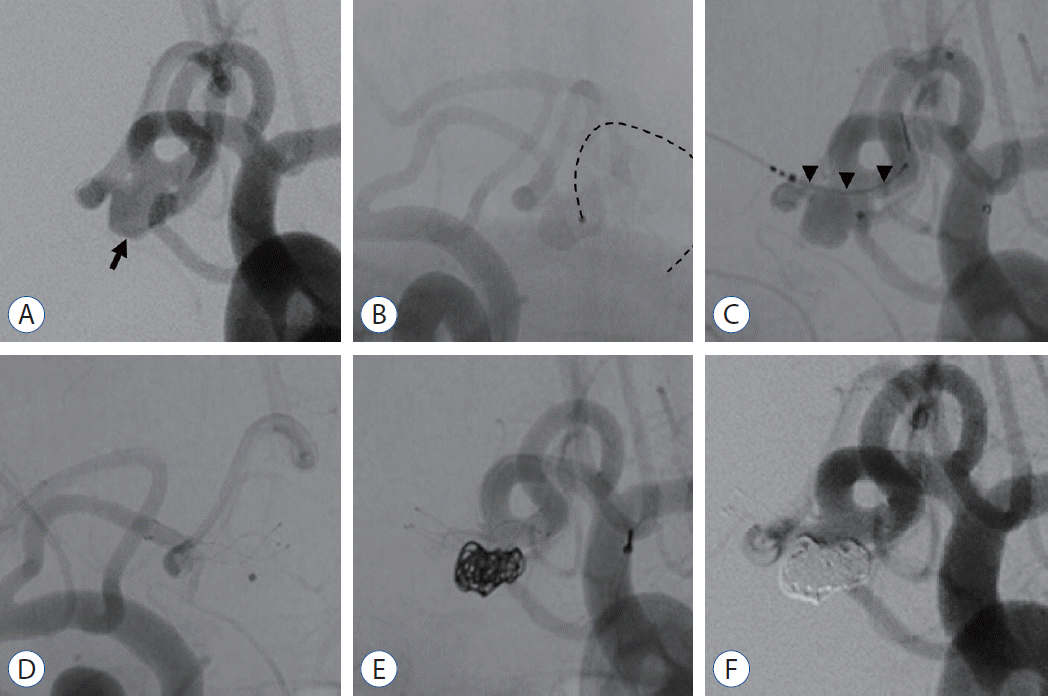
Fig. 3.
. A case of periprocedural thromboembolic complication. A : After stent deployment, right internal carotid artery angiography showed patency of nondominant A1 and ipsilateral A2 blood flow (arrows). B : Packing of bare platinum coils into the aneurysm. C : Immediate post-DSA showed that the aneurysm was completely occluded without complications. D : On the 6-hour follow-up diffusion-weighted image, there were multiple scattered infarctions in the left ACA-MCA border zone area (arrowheads). E : On the 6-hour follow-up DSA, there was no definite thrombus formation around the stented vessels. F : Occlusion of the left small ACA cortical branches was detected (arrows). DSA : digital subtraction angiography, ACA : anterior cerebral
artery, MCA : middle cerebral artery.
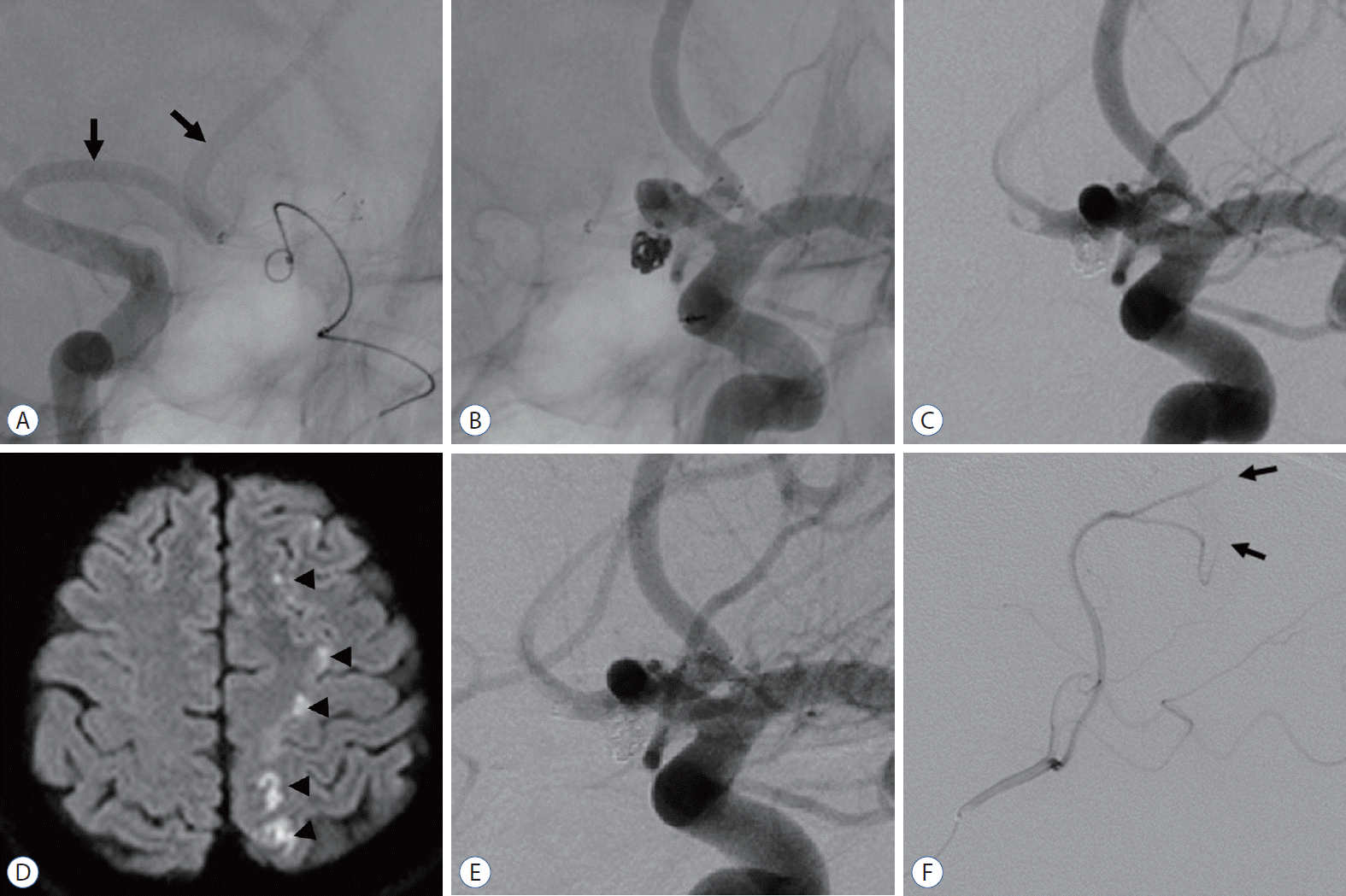
Table 1.
Summary of the characteristics of patients and aneurysms
Table 2.
Outcomes of patients
| No. | Age (years)/sex | Initial mRS score | Degree of initial obliteration* | Complication | Clinical FU period (months) | Last mRS score | Radiological FU period (months) | Patency of stented vessels on FU image* | Degree of last FU obliteration |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 52/F | 0 | 1 | None | 51 | 0 | 51 | Patent | 1† |
| 2 | 78/F | 0 | 3 | None | 45 | 0 | 45 | - | 1 |
| 3 | 68/F | 0 | 1 | None | 46 | 0 | 41 | Patent | 1 |
| 4 | 69/F | 0 | 1 | None | 46 | 0 | 46 | Patent | 1 |
| 5 | 59/F | 0 | 1 | None | 48 | 0 | 48 | - | 1 |
| 6 | 62/M | 1 | 2 | None | 43 | 1 | 32 | Patent | 2‡ |
| 7 | 68/M | 1 | 1 | None | 48 | 1 | 48 | Patent | 1 |
| 8 | 69/F | 0 | 1 | None | 28 | 0 | 28 | Patent | 1 |
| 9 | 68/F | 0 | 1 | None | 46 | 0 | 46 | Patent | 1 |
| 10 | 62/M | 0 | 1 | None | 47 | 0 | 47 | - | 1 |
| 11 | 44/M | 0 | 1 | None | 30 | 0 | 30 | - | 1 |
| 12 | 73/M | 0 | 1 | None | 45 | 0 | 45 | - | 1 |
| 13 | 59/M | 0 | 1 | None | 22 | 0 | 22 | - | 1 |
| 14 | 61/F | 1 | 1 | None | 40 | 1 | 40 | - | 1 |
| 15 | 71/F | 0 | 1 | None | 22 | 0 | 22 | Patent | 1 |
| 16 | 62/F | 0 | 1 | Thromboembolic | 31 | 0 | 31 | Patent | 1 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download