Abstract
Various grading systems and surgical techniques have been developed for the treatment of intraventricular hemorrhage (IVH); however, little attention has been paid to the fourth ventricle hematoma. Nonetheless, hemorrhagic dilation of the fourth ventricle may lead to catastrophic consequences for patients with massive IVH. We present two cases of massive IVH accompanied by massive fourth ventricle hematoma which was successfully removed with combination of suboccipital craniotomy for fourth ventricle hematoma and intraventricular fibrinolysis for supratentorial hematoma.
Intraventricular hemorrhage (IVH) is defined as bleeding into the intracranial ventricular system. It is classified as primary IVH when the bleeding originates from intraventricular sources or lesions and secondary IVH when a periventricular lesion (lesion in the putamen or thalamus, an aneurysm, or a vascular malformation) bleeds and ruptures into the ventricle [4,10,15]. Regardless of its etiology, IVH is associated with sudden neurological deterioration or development of coma owing to the increased intracranial pressure (ICP), mass effect on the brain tissue, and blockage of the circulation of the cerebrospinal fluid, leading to acute hydrocephalus [5,17].
The prognosis of IVH is very poor in the literature, with morbidity and mortality rates of up to 70% and 80%, respectively. Among those with massive flooding and marked dilation of the ventricles, the worst outcomes and highest mortality rates have been observed [5]. Hence, massive IVH is a neurosurgical emergency and should be treated with the prompt removal of intraventricular blood for rapid reversal of ventricular dilation and normalization of ICP.
Recently, reports on the endoscopic removal of hematomas have shown better survival rates and prognoses compared with traditional surgical approaches, such as external ventricular drainage (EVD), EVD with intraventricular fibrinolysis, and hematoma evacuation by craniotomy [1,11,14]. However, most studies have focused on supratentorial IVH (lateral horn, occipital horn, and third ventricle), and little attention has been given to the presence of blood in the fourth ventricle. Supratentorial IVH is important, but consideration should also be given to hematomas filling the fourth ventricle due to the significant mass effect on the brainstem and surrounding ependyma.
Herein, we present two cases of massive IVH that were treated with emergent evacuation of fourth ventricle hematoma following EVD.
An 18-year-old woman presented in an unconscious state with a Glasgow coma scale (GCS) rating of 4. The motor response to painful stimuli was decerebration, and both pupils were pinpointed. Computed tomography (CT) scan revealed massive IVH extending from the lateral ventricle to the fourth ventricle accompanied by hydrocephalus. The fourth ventricle was clearly enlarged by the hematoma, and there was a significant mass effect on the brainstem and the posterior fossa cisterns. (Fig. 1A and B) Bilateral ventricular catheters were promptly inserted to control the ICP. Subsequent digital subtraction angiography revealed a forniceal arteriovenous malformation with an intranidal aneurysm measuring 6 mm in size. The aneurysm was successfully embolized using Histoacryl® (B. Braun, Melsungen, Germany) (Fig. 1C and D). Although the hydrocephalus was somewhat relieved, the patient’ s condition did not improve further. Consequently, we considered the possibility that there may be significant mass effect in the brainstem owing to the fourth ventricle hematoma. Immediately after embolization, suboccipital craniotomy and evacuation of the fourth ventricle hematoma were performed. After opening the cerebellomedullary cistern, the cerebellomedullary fissure was sharply dissected, and the cerebellar tonsils were elevated using a retractor. A large amount of the blood clot filling the fourth ventricle was removed, and care was taken not to irritate the brainstem (Fig. 2A and B). After removing the clot, the brainstem and cerebellum became relaxed. Postoperative CT scan showed complete removal of the hematoma with visible posterior fossa cisterns (Fig. 2C and D). Immediately after surgery, she recovered to the point of localizing pain in her upper extremities. For clot lysis in the lateral and third ventricles, 1 mg of intraventricular tissue plasminogen activator (tPA) was administrated into each ventricular catheter. A total of eight doses of tPA were administered over the course of 4 consecutive days. The patient was gradually stabilized and began to follow simple commands. A follow-up CT scan showed near-total resolution of the IVH, and a ventriculoperitoneal shunt was inserted at 3 weeks after the hemorrhage (Fig. 3). Three months after the of rehabilitation, the patient had moderately severe disability (modified Rankin Score of 4 points); however, she was still able to communicate with her family.
A 60-year-old woman presented with impaired consciousness. The GCS rating at admission was 8, and she showed weak bilateral localization upon exposure to painful stimuli. CT scan revealed a massive IVH with hydrocephalus and dilatation of the fourth ventricle (Fig. 4A and B). Emergent bilateral ventriculostomy was performed in order to relieve the hydrocephalus. As in the first case, although the hydrocephalus was improved, the neurological condition remained unchanged. Likewise, it was thought that there could be significant compression of the brainstem by the fourth ventricle hematoma. Suboccipital craniotomy and evacuation of the fourth ventricle hematoma were subsequently performed. The cerebellomedullary fissure was dissected, and the cerebellar tonsils were gently elevated with the help of a retractor. We removed a large amount of the blood clot filling the fourth ventricle, taking care not to touch the brainstem in the process (Fig. 4C and D). After removing the blood clot, relaxation of the brainstem and cerebellum was observed. A postoperative CT scan showed that the hematoma had been completely removed and that the hydrocephalus had improved. Immediately after surgery, the patient recovered to the point of being able to obey basic commands. We administered 1 mg of intraventricular tPA into each of the ventricular catheters for clot lysis. Six doses of tPA were administered over a span of three consecutive days, and a follow-up CT scan revealed that near-total resolution of the intraventricular blood had been achieved (Fig. 5). The patient recovered well (modified Rankin Score of 1) and was discharged without requiring further shunt surgery (Fig. 6).
Irrespective of the etiology, neurosurgeons often face a dilemma when confronted with massive IVH. Specifically, their choices in terms of treatment include EVD, intraventricular fibrinolysis, endoscopic evacuation, and craniotomy. In any case, it is clear is that the treatment goals of massive IVH should be the removal of intraventricular blood, rapid reversal of ventricular dilatation, and normalization of ICP.
Massive IVH is often accompanied by fourth ventricle hematoma. When the fourth ventricle dilates as a result of an intraventricular clot, the brainstem can undergo compression. If persistent, potential outcomes may include a decreased level of consciousness or death. So, similarly to cerebellar hemorrhage, massive fourth ventricular hematoma demands prompt decompression when a tight posterior fossa, brainstem compression, or basal cistern compression is observed [2,16]. Several widely-accepted grading systems have been used to estimate the severity of IVH and its prognosis. These include the Graeb, modified Graeb, and LeRoux grading systems [6,8,12]. The score of each grading system is a sum of the compartment scores that are given to the lateral, third, and fourth ventricles. However, the scores of these grading systems have been found to be skewed mainly in favor of the lateral ventricles. As previously stated, attention should also be paid to hematomas of the fourth ventricle.
With a rising interest in minimally-invasive techniques, endoscopic surgery and EVD with intraventricular fibrinolysis have come to be widely accepted. Compared to EVD alone, there is growing evidence to support the notion that endoscopic surgery and EVD in conjunction with intraventricular fibrinolysis can yield better results in terms of efficacy and safety in patients with massive IVH [9,11,14]. In particular, endoscopic surgery offers many advantages, including a high evacuation rate and low incidences of rebleeding, shunt dependency, and infection [11]. A rigid endoscope is typically used due to the high quality of view; however, it is limited in its ability to remove hematomas in the fourth ventricle. Compared with the rigid endoscope, a flexible endoscope offers higher degrees of flexibility and accessibility when evacuating hematomas in the aqueduct and fourth ventricle [7]. Nonetheless, flexible endoscopes have a poorer quality of vision, a limitation that can cause injury to deep structures. This consideration is particularly pertinent given that the fourth ventricle is the deepest portion of the ventricular system [3,7]. Moreover, the endoscope occupies the entire diameter of the cerebral aqueduct, thereby disturbing irrigation. Excessive irrigation has been found to cause local hypertension and bradycardia in prior reports [7]. Hence, if massive blood exists in the fourth ventricle, a finding that requires prompt decompression, the use of a flexible endoscope is relatively time-consuming. In fact, brainstem infarction has been observed to occur more rapidly than previously thought, impairing perforator perfusion [13]. Upon gross examination, autopsy has previously revealed medullopontine softening while histology has found multiple pontine microinfarcts [13].
In our two cases, we took great care of unchanged neurological symptoms despite ventricular drainage, which suggests that the compartmentalization of ICP occurred between the supratentorial and infratentorial regions. Hence, we decided to conduct the treatment with a combination of EVD and fibrinolysis (for supratentorial clot removal) and hematoma evacuation with craniotomy (for infratentorial clot removal). Visible relaxation and pulsation of the posterior fossa was observed following removal of the clot, potentially indicating that fourth ventricle hematoma could be a major contributor to decreased levels of consciousness.
Although, it is debatable to conclude with only two cases that our method is better over the intraventricular fibrinolysis or other minimally-invasive techniques, we should consider whether the mass effect on posterior fossa is the main cause of decreased consciousness in patients with massive fourth ventricular hematoma and surgical evacuation could be an useful option in these conditions.
Notes
ACKNOWLEDGMENTS
This study was supported by a grant (BCRI20039) of Chonnam National University Hospital Biomedical Research Institute.
References
1. Di Rienzo A, Colasanti R, Esposito D, Della Costanza M, Carrassi E, Capece M, et al. Endoscope-assisted microsurgical evacuation versus external ventricular drainage for the treatment of cast intraventricular hemorrhage: results of a comparative series. Neurosurg Rev. 43:695–708. 2020.

2. Donauer E, Loew F, Faubert C, Alesch F, Schaan M. Prognostic factors in the treatment of cerebellar haemorrhage. Acta Neurochir (Wien). 131:59–66. 1994.

3. Feletti A, Basaldella L, Fiorindi A. How I do it: flexible endoscopic aspiration of intraventricular hemorrhage. Acta Neurochir (Wien). 162:3141–3146. 2020.

4. Findlay JM, Grace MG, Weir BK. Treatment of intraventricular hemorrhage with tissue plasminogen activator. Neurosurgery. 32:941–947. discussion 947. 1993.

5. Gaberel T, Magheru C, Emery E. Management of non-traumatic intraventricular hemorrhage. Neurosurg Rev. 35:485–494. discussion 494-495. 2012.

6. Graeb DA, Robertson WD, Lapointe JS, Nugent RA, Harrison PB. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology. 143:91–96. 1982.

7. Hamada H, Hayashi N, Kurimoto M, Umemura K, Nagai S, Kurosaki K, et al. Neuroendoscopic removal of intraventricular hemorrhage combined with hydrocephalus. Minim Invasive Neurosurg. 51:345–349. 2008.

8. LeRoux PD, Haglund MM, Newell DW, Grady MS, Winn HR. Intraventricular hemorrhage in blunt head trauma: an analysis of 43 cases. Neurosurgery. 31:678–684. discussion 684-685. 1992.
9. Li Y, Zhang H, Wang X, She L, Yan Z, Zhang N, et al. Neuroendoscopic surgery versus external ventricular drainage alone or with intraventricular fibrinolysis for intraventricular hemorrhage secondary to spontaneous supratentorial hemorrhage: a systematic review and meta-analysis. PLoS One. 8:e80599. 2013.

10. Martí-Fàbregas J, Piles S, Guardia E, Martí-Vilalta JL. Spontaneous primary intraventricular hemorrhage: clinical data, etiology and outcome. J Neurol. 246:287–291. 1999.

11. Mei L, Fengqun M, Qian H, Dongpo S, Zhenzhong G, Tong C. Exploration of efficacy and safety of interventions for intraventricular hemorrhage: a network meta-analysis. World Neurosurg. 136:382–389.e6. 2020.
12. Morgan TC, Dawson J, Spengler D, Lees KR, Aldrich C, Mishra NK, et al. The modified Graeb score: an enhanced tool for intraventricular hemorrhage measurement and prediction of functional outcome. Stroke. 44:635–641. 2013.
13. Shapiro SA, Campbell RL, Scully T. Hemorrhagic dilation of the fourth ventricle: an ominous predictor. J Neurosurg. 80:805–809. 1994.

14. Song P, Duan FL, Cai Q, Wu JL, Chen XB, Wang Y, et al. Endoscopic surgery versus external ventricular drainage surgery for severe intraventricular hemorrhage. Curr Med Sci. 38:880–887. 2018.

15. Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 27:617–621. 1999.

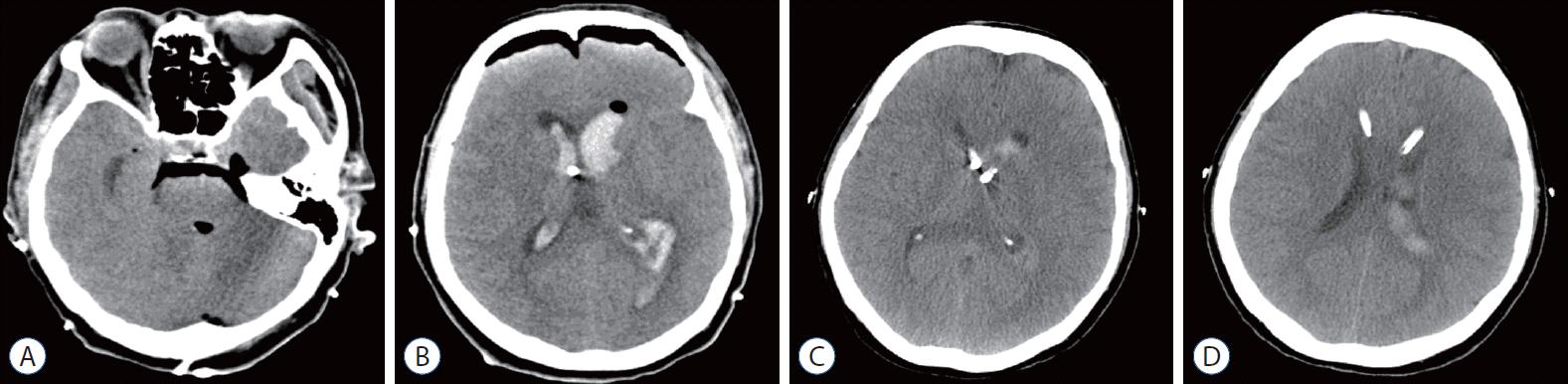
Fig. 1.
Case 1. A and B : Initial computed tomography scan showed massive intraventricular hemorrhage with a fourth ventricle hematoma compressing the brainstem. C and D : The intranidal aneurysm was successfully obliterated in the forniceal arteriovenous malformation using Histoacryl® (B. Braun, Melsungen, Germany). Thick arrow indicates intranidal aneurysm.
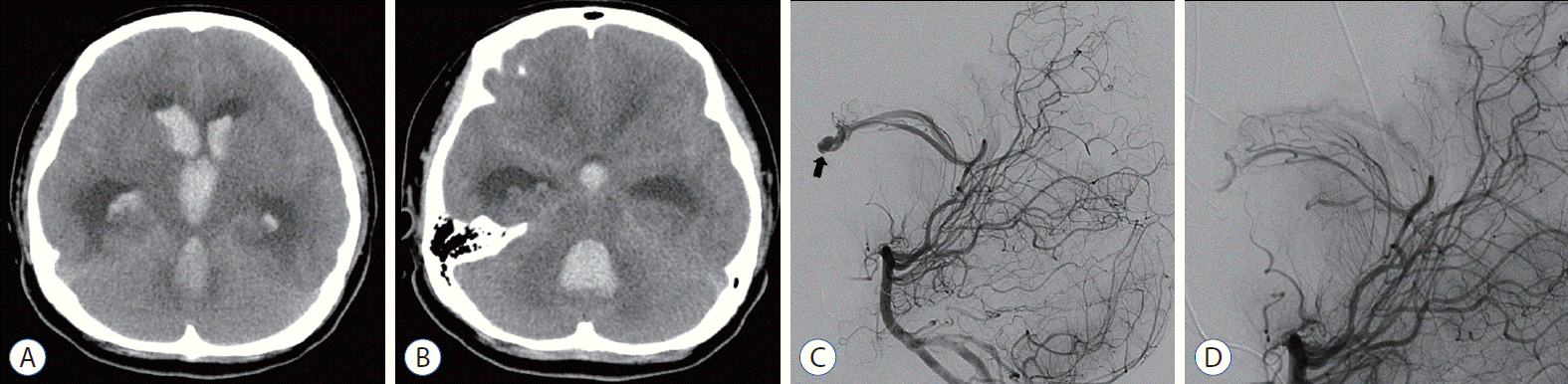
Fig. 2.
Case 1. A and B : Removal of fourth ventricle hematoma was performed via midline suboccipital craniotomy. C and D : Postoperative computed tomography scan revealed complete removal of the fourth ventricle hematoma and slight improvement of the hydrocephalus.
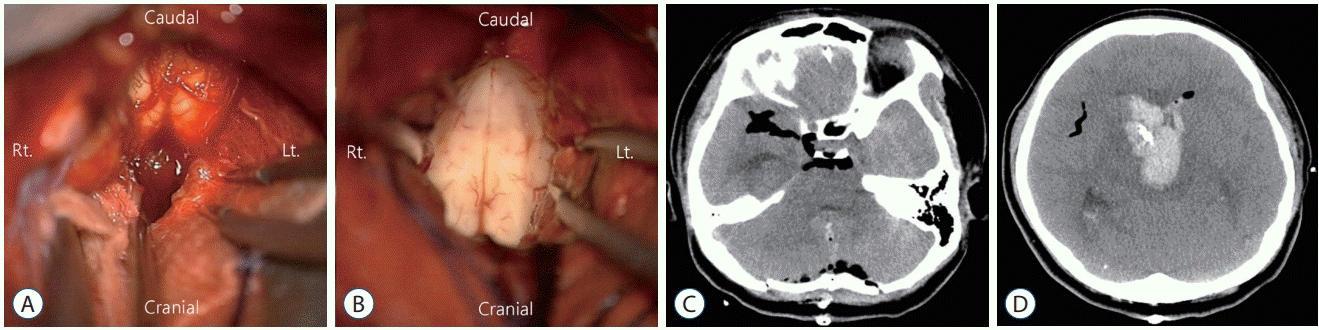
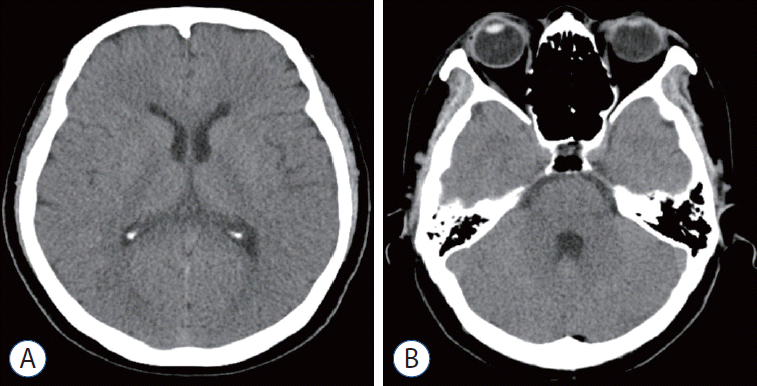
Fig. 3.
Case 1. A : The intraventricular hemorrhage was almost completely resolved following eight doses of intraventricularly administered tissue plasminogen activator given over 4 consecutive days. B : Normal ventricle size was maintained by a shunt during follow-up computed tomography scan at 18 months after surgery.
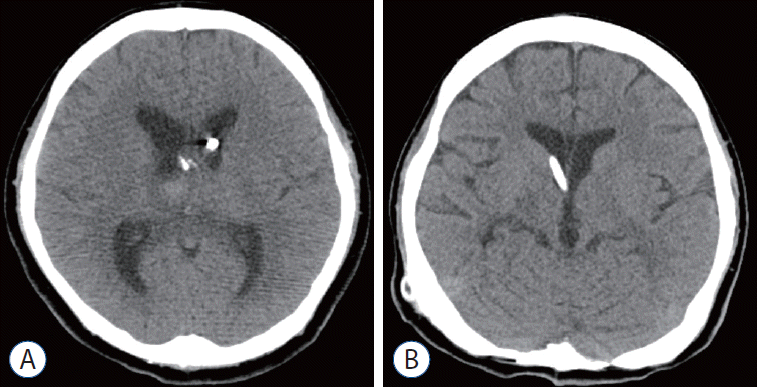
Fig. 4.
Case 2. A and B : An initial computed tomography scan revealed massive intraventricular hemorrhage with a fourth ventricle hematoma compressing the brainstem. C and D : The fourth ventricle hematoma was removed via midline suboccipital craniotomy.
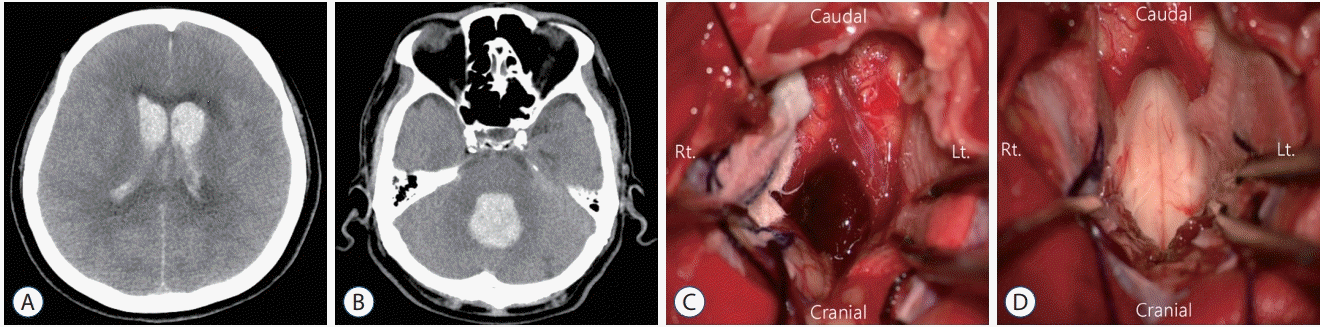
Fig. 5.
Case 2. A and B : A postoperative computed tomography scan showed complete removal of the fourth ventricle hematoma and improvement of the hydrocephalus. C and D : The intraventricular hemorrhage was almost completely resolved following six doses of intraventricularly administered tissue plasminogen activator given over 3 consecutive days.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download