Abstract
Objective
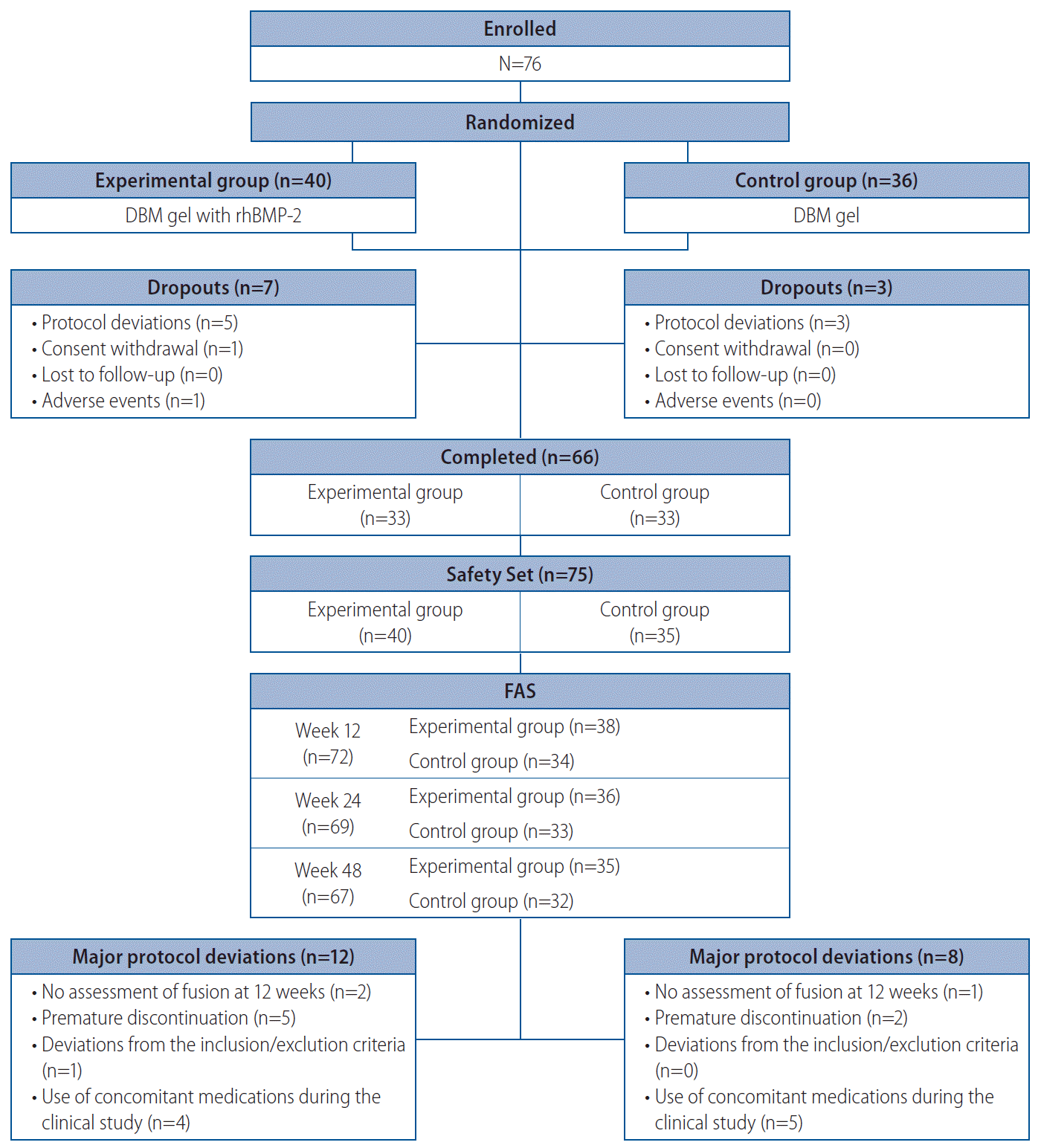
Methods
Results
Conclusion
Notes
CONFLICTS OF INTEREST
Seung Hwan Yoon has been editorial board of JKNS since November 2016. He was not involved in the review process of this original article. No potential conflict of interest relevant to this article was reported.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in this study.
AUTHOR CONTRIBUTIONS
Conceptualization : SJH, YH
Data curation : SJH, SHY, JHK, JKO, YH
Formal analysis : CHL, JJS, JK
Funding acquisition : SJH, SHY, JHK, JKO, YH
Methodology : SJH, SHY, JHK, JKO, YH
Project administration : YH
Visualization : SJH
Writing - original draft : SJH
Writing - review & editing : YH
ACKNOWLEDGMENTS
References
Table 1.
Table 2.
| Parameter | DBM gel with rhBMP-2 (n=40) | DBM gel (n=36) | p-value |
|---|---|---|---|
| Sex, M/F | 23/17 (57.50/42.50) | 19/17 (52.78/47.22) | 0.6793* |
| Age (years) | 64.03 (10.63) | 62.67 (9.32) | 0.5573† |
| Height (cm) | 162.13 (7.89) | 162.75 (10.10) | 0.7633† |
| Weight (kg) | 63.77 (8.25) | 69.20 (11.84) | 0.0251† |
| BMI (kg/m2) | 24.27 (2.73) | 26.16 (4.19) | 0.0243† |
| BMD, T-score | -0.55 (1.38) | -0.43 (1.58) | 0.7274† |
| Smoker | 25/15 (62.50/37.50) | 25/11 (69.44/30.55) | 0.6920‡ |
| Drug abuse | 40/0 (100.00/0.00) | 36/0 (100.00/0.00) | NA |
| Level affected by DDD | 40 | 36 | 0.3214‡ |
| L3–L4 | 3 (7.50) | 5 (13.89) | |
| L4–L5 | 18 (45.00) | 10 (27.78) | |
| L5–S1 | 4 (10.00) | 4 (11.11) | |
| L3–L4 & L4–L5 | 10 (25.00) | 8 (22.22) | |
| L4–L5 & L5–S1 | 4 (10.00) | 9 (25.00) | |
| L3–L4 & L4–L5 & L5–S1 | 1 (2.50) | 0 (0.00) |
‡ A Fisher’s exact test was used for comparison between groups in categorical variables. DBM : demineralized bone matrix, rhBMP-2 : recombinant human bone morphogenetic protein-2, M : male, F : female, BMI : body mass index, BMD : bone mineral density, NA : not applicable, DDD : degenerative disc disease, N : number of observed subjects, number of subjects corresponding to each parameter
Table 3.
| DBM gel with rhBMP-2 (n=40) | DBM gel (n=35) | Treatment difference p-value | |||
|---|---|---|---|---|---|
| Fusion rate | |||||
| Fusion rate at 12 weeks | 38 | 34 | |||
| Fusion | 28 (73.68) | 20 (58.82) | 0.1817* | ||
| Non-fusion | 10 (26.32) | 14 (41.18) | |||
| Fusion rate at 24 weeks | 36 | 33 | |||
| Fusion | 26 (72.22) | 26 (78.79) | 0.5272* | ||
| Non-fusion | 10 (27.78) | 7 (21.21) | |||
| Fusion rate at 48 weeks | 35 | 32 | |||
| Fusion | 29 (82.86) | 25 (78.13) | 0.6247* | ||
| Non-fusion | 6 (17.14) | 7 (21.88) | |||
| 100-mm VAS pain scores | |||||
| Baseline | 72.08±16.45 (n=40) | 74.43±13.90 (n=35) | |||
| 12 weeks | 26.11±20.60 (n=38) | 37.56±27.15 (n=34) | |||
| 24 weeks | 25.47±21.96 (n=36) | 31.00±20.74 (n=33) | |||
| 48 weeks | 22.26±19.92 (n=35) | 24.50±18.12 (n=32) | |||
| 12 weeks–baseline | -45.45±27.84 (n=38) | -36.97±31.78 (n=34) | |||
| p-value | <0.0001† | <0.0001† | 0.2316‡ | ||
| 24 weeks–baseline | -45.31±26.79 (n=36) | -43.39±27.81 (n=33) | |||
| p-value | <0.0001† | <0.0001† | 0.7721‡ | ||
| 48 weeks–baseline | -48.00±26.07 (n=35) | -50.47±23.38 (n=32) | |||
| p-value | <0.0001† | <0.0001† | 0.6856‡ | ||
| ODI QOL scores | |||||
| Baseline | 46.91±16.65 (n=40) | 51.03±13.16 (n=35) | |||
| 12 weeks | 26.41±16.44 (n=38) | 33.08±18.37 (n=34) | |||
| 24 weeks | 24.61±16.22 (n=36) | 30.77±16.56 (n=33) | |||
| 48 weeks | 22.28±17.13 (n=35) | 26.33±17.19 (n=32) | |||
| 12 weeks–baseline | -20.34±19.99 (n=38) | -18.62±22.90 (n=34) | |||
| p-value | <0.0001† | <0.0001† | 0.7349‡ | ||
| 24 weeks–baseline | -22.96±17.11 (n=36) | -20.81±20.35 (n=33) | |||
| p-value | <0.0001† | <0.0001† | 0.6346‡ | ||
| 48 weeks–baseline | -25.11±21.35 (n=35) | -25.36±18.82 (n=32) | |||
| p-value | <0.0001† | <0.0001† | 0.9598‡ | ||
Table 4.
Table 5.
| DBM gel with rhBMP-2 (n=40) | DBM gel (n=35) | p-value | |
|---|---|---|---|
| ADE | 6 (15.00) | 8 (22.86) | 0.3836* |
| Mild | 5 (12.50) | 6 (17.14) | |
| Moderate | 0 (0.00) | 2 (5.71) | |
| Severe | 1 (2.50) | 0 (0.00) | |
| SADE related to the investigational device | 2 (5.00) | 3 (8.57) | 0.6594† |
| Relatedness to the investigational device | |||
| Unrelated | 0 (0.00) | 0 (0.00) | |
| Unlikely | 5 (12.50) | 8 (22.86) | |
| Possible | 1 (2.50) | 0 (0.00) | |
| Probable | 0 (0.00) | 0 (0.00) | |
| Certain | 0 (0.00) | 0 (0.00) | |
| SOC PT | |||
| General disorders and administration site conditions | 2 (5.00) | 3 (8.57) | |
| Pyrexia | 2 (5.00) | 1 (2.86) | |
| Chest discomfort | 0 (0.00) | 1 (2.86) | |
| Pain | 0 (0.00) | 1 (2.86) | |
| Nervous system disorders | 3 (7.50) | 0 (0.00) | |
| Hypesthesia | 1 (2.50) | 0 (0.00) | |
| Paresthesia | 1 (2.50) | 0 (0.00) | |
| Transient peripheral paralysis | 1 (2.50) | 0 (0.00) | |
| Musculoskeletal and connective tissue disorders | 1 (2.50) | 1 (2.86) | |
| Osteoarthritis | 0 (0.00) | 1 (2.86) | |
| Spondylitis | 1 (2.50) | 0 (0.00) | |
| Renal and urinary disorders | 0 (0.00) | 2 (5.71) | |
| Nephropathy toxic | 0 (0.00) | 1 (2.86) | |
| Neurogenic bladder | 0 (0.00) | 1 (2.86) | |
| Investigations | 0 (0.00) | 1 (2.86) | |
| Liver function analyses | 0 (0.00) | 1 (2.86) | |
| Psychiatric disorders | 1 (2.50) | 0 (0.00) | |
| Insomnia | 1 (2.50) | 0 (0.00) | |
| Skin and subcutaneous tissue disorders | 0 (0.00) | 1 (2.86) | |
| Urticaria | 0 (0.00) | 1 (2.86) | |
| SADE | 2 (5.00) | 3 (8.57) | 0.6446‡ |
| General disorders and administration site conditions | 1 (2.50) | 1 (2.86) | |
| Chest discomfort | 0 (0.00) | 1 (2.86) | |
| Pyrexia | 1 (2.50) | 0 (0.00) | |
| Musculoskeletal and connective tissue disorders | 1 (2.50) | 1 (2.86) | |
| Osteoarthritis | 0 (0.00) | 1 (2.86) | |
| Spondylitis | 1 (2.50) | 0 (0.00) | |
| Renal and urinary disorders | 0 (0.00) | 1 (2.86) | |
| Neurogenic bladder | 0 (0.00) | 1 (2.86) |
ADEs : adverse device effects, SADEs : serious adverse device effects, FAS : full analysis set, DBM : demineralized bone matrix, rhBMP-2 : recombinant human bone morphogenetic protein-2, n : number of observed subjects number of subjects corresponding to each category, SOC : system organ class, PT : preferred term




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download