Abstract
Objective
Methods
Results
Supplementary Materials
Supplementary Table 1.
References
Fig. 1.
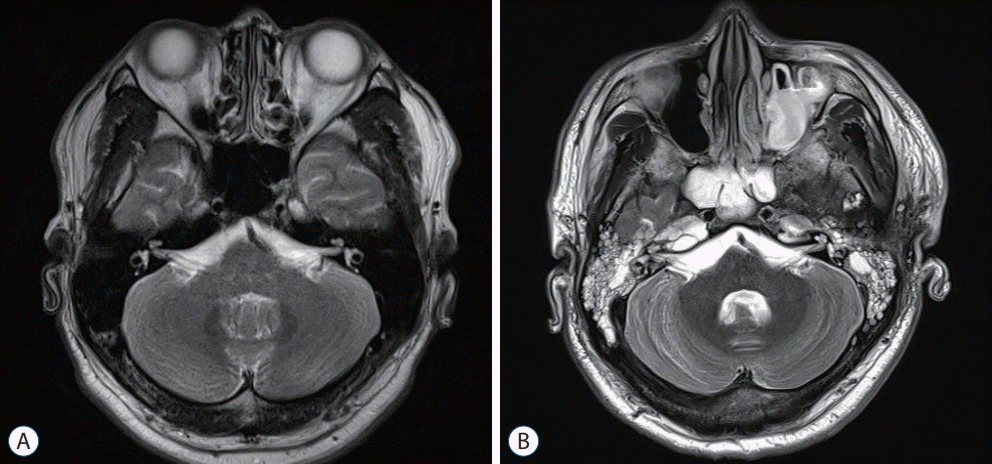
Fig. 2.
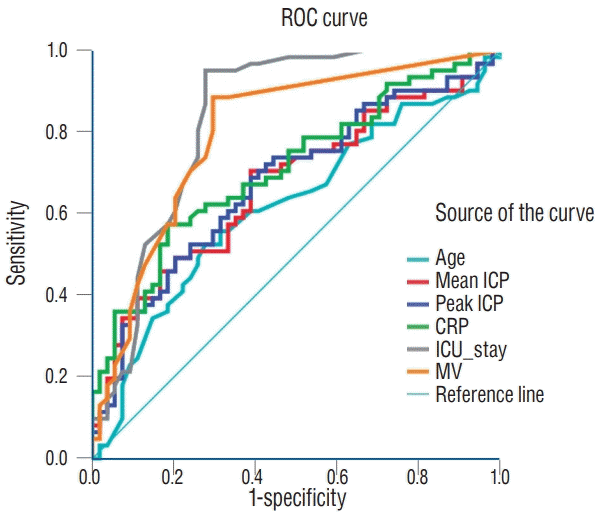
Table 1.
Values are presented as mean±standard deviation or number (%). ME : mastoid effusion, SAH : subarachnoid hemorrhage, ICH : intracerebral hemorrhage, IVH : intraventricular hemorrhage, ICP : intracranial pressure, CRP : C-reactive protein, ICU : intensive care unit, ETT : endotracheal tube, NGT : nasogastric tube
Table 2.
Table 3.
Table 4.
| Variable | Model A* | Model B† | Model C‡ |
|---|---|---|---|
| Mean ICP | 1.113 (1.018–1.218) | ||
| Peak ICP | 1.114 (1.024–1.213) | ||
| Sex | 0.392 (0.111–1.391) | 0.284 (0.076–1.063) | 0.271 (0.072–1.023) |
| Trauma | 4.160 (1.076–16.091) | 4.554 (1.064–19.490) | 4.798 (1.084–21.242) |
| Operation | |||
| Craniotomy vs. craniostomy | 0.144 (0.023–0.889) | 0.095 (0.013–0.715) | 0.087 (0.011–0.679) |
| Craniectomy vs. craniostomy | 0.213 (0.037–1.243) | 0.129 (0.017–0.995) | 0.129 (0.016–1.022) |
| CRP | 1.008 (1.001–1.016) | 1.010 (1.002–1.018) | 1.010 (1.002–1.018) |
| ICU stay | 1.056 (1.004–1.111) | 1.058 (1.003–1.115) | 1.062 (1.007–1.119) |
| Presence of ETT | 7.510 (1.834–30.754) | 4.912 (1.140–21.172) | 4.578 (1.058–19.808) |
| Presence of NGT | 2.572 (0.704–9.399) | 3.639 (0.848–15.624) | 3.490 (0.814–14.970) |
* In a model A, which included sex, trauma, operation method, CRP, ICU stay and presence of ETT and NGT whereas excluded mean ICP and maximal ICP, prediction of estimation was significant (AUC, 0.908; 95% CI, 0.856–0.96; p<0.001).
† With a model B, in which mean ICP was a fixed variable and other variables in model A were eliminated backward step. Model B in prediction of estimation was significant (AUC, 0.923; 95% CI, 0.877–0.968; p<0.001). When mean ICP increases as 1, development of ME increases 11.3%.
‡ With a model C, in which peak ICP was a fixed variable and other variables in model A were eliminated backward step. Model C in prediction of estimation was significant (AUC, 0.925; 95% CI, 0.88–0.97; p<0.001). When maximal ICP increases as 1, development of ME increases 11.4%. ICP : intracranial pressure, CRP : C-reactive protein, ICH : intracerebral hemorrhage, ETT : endotracheal tube, NGT : nasogastric tube, CI : confidence interval, AUC : area under the curve, ME : mastoid effusion




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download