Abstract
Objective
Spinal epidural abscess (SEA) is a severe and life-threatening disease. Although commonly performed, the effect of timing in surgical treatment on patient outcome is still unclear. With this study, we aim to provide evidence for early surgical treatment in patients with SEA.
Methods
Patients treated for SEA in the authors’ department between 2007 and 2016 were included for analysis and retrospectively analyzed for basic clinical parameters and outcome. Pre- and postoperative neurological status were assessed using the American Spinal Injury Association Impairment Scale (AIS). The self-reported quality of life (QOL) based on the Short-Form Health Survey 36 (SF-36) was assessed prospectively. Surgery was defined as “early”, when performed within 12 hours after admission and “late” when performed thereafter. Conservative therapy was preferred and recommend in patients without neurological deficits and in patients denying surgical intervention.
Results
One hundred and twenty-three patients were included in this study. Forty-nine patients (39.8%) underwent early, 47 patients (38.2%) delayed surgery and 27 (21.9%) conservative therapy. No significant differences were observed regarding mean age, sex, diabetes, prior history of spinal infection, and bony destruction. Patients undergoing early surgery revealed a significant better clinical outcome before discharge than patients undergoing late surgery (p=0.001) and conservative therapy. QOL based on SF-36 were significantly better in the early surgery cohort in two of four physical items (physical functioning and bodily pain) and in one of four psychological items (role limitation) after a mean follow-up period of 58 months. Readmission to the hospital and failure of conservative therapy were observed more often in patients undergoing conservative therapy.
Spinal epidural abscess (SEA) is a severe disease, which may have enormous implication on patient’s outcome and health related satisfaction [4-8,10,12,16-18]. Despite improvement in medical knowledge, technical and surgical techniques the management of SEA still remains challenging [2,15,31,33-36]. The total incidence of the disease amounts 2–3 cases per 10000 admissions. The average age at admission is above 50 years with a male predomination [8,29,33]. The most common abscess location is the lumbar spine, followed by the thoracic spine and the cervical spine [1,4,6,20,24]. Patients with severe pain or a neurological deficit, such as dysesthesia or motor weakness mostly undergo early surgery. However, controversial data have been published on the optimal timing for surgical treatment of SEA. More specifically, the literature disagrees on the beneficial effect of early surgery on SEA [3,15,25,30,32,35,36]. The aim of this study is to evaluate both clinical outcome and health-related quality of life (QOL) in patients following early versus late surgical treatment for SEA.
This study was approved by the Local Ethics Committee of Goethe University Frankfurt. A retrospective analysis was performed for all cases treated for SEA between 2007–2016. Patient charts were assessed for clinical, surgical, radiological and microbiological data. The neurological status was assessed using the American Spinal Injury Association Impairment Scale (AIS). This assessment was performed before surgery, postoperatively and at the time of discharge. According to our treatment algorithm, magnetic resonance imaging and computed tomography (CT) scans were performed to confirm the diagnosis, extent of infection and bony destruction. Indication for surgery were compression of nervous structures causing neurological deficits or severe pain, bony destruction and obvious signs of instability. Based on the clinical status at first presentation, the procedure was carried out either as an emergency surgery or as an elective surgery after admission. In this study we chose to evaluate the impact of early surgical treatment with an even shorter interval than 24 hours after admission being defined as 12 hours after admission. Early surgery was defined as surgery being performed within 12 hours after admission, late surgery was defined when the procedure was performed 12 hours after admission. Patients with severe neurological deficits, intractable pain and obvious predominant osteomyelitis or spondylodiscitis were assigned to early surgery group, whereas patients with mild symptoms, no symptoms and evident onset of neurological deficits longer than 72 days were assigned to the delayed surgery group. Thus, the onset of the symptoms and neurological deficits was another important factor for performing early or delayed surgery. Further, most reasons for delayed surgery was either failure of nonoperative treatment, preoperative work up and evaluation of the physical condition in cases of multimorbidity and delay in diagnosis.
Conservative therapy was either chosen by patients itself refusing surgery, harboring comorbidities with increased risk for perioperative complications or revealing no neurological deficits.
In the further follow-up period most patients with AIS E (mild or no symptoms) underwent surgery in order of severe pain and proven increase of bony destruction. Samples for bacteriological work-up were obtained by blood culture, during surgery or by CT-guided biopsy in conservatively treated patients. Calculated broad-spectrum antibiotics were administered until receiving the result of microbiological examination. Based on the result of the individual antibiogram the broad-spectrum antibiotics therapy was deescalated to pathogen specific antibiotics. The antibiotic regimen was routinely applied as intravenous treatment for 6 weeks followed by 6 weeks of oral antibiotics. This regimen was individually adapted to the microbiological findings, comprising of a minimum of 12 weeks’ total antibiotic therapy. The treatment was discontinued when C-reactive protein (CRP) and whole blood count normalized and clinical as well as radiological evaluation showed improvement. Surgical procedure ranged from open drainage, debridement and decompression of spinal cord and/or dura by laminectomy, hemilaminectomy, extended fenestration alone or debridement and placement of instrumentation. Instrumentation of the spine included dorsal, ventral or combined approaches. Postoperative pain management was carried out as published before [14]. QOL was evaluated prospectively with the Short-Form Health Survey 36 (SF-36) questionnaire. Intergroup comparison for detection of differences regarding patient´s QOL were performed in the follow up. Despite recommendation for early surgery, some patients denied surgery even in cases of radiologically evident osteomyelitis and favored conservative therapy with admission of antibiotics and underwent nonsurgical therapy.
QOL was assessed with the SF-36 (Supplementary Table 1). The SF-36 was converted in an eight-item scale, which was divided into two categories, physical and psychological health. For categorical variables Fischer´s exact test was used. Unpaired t test was used for parametric statistics. Results with a p value <0.05 were considered statistically significant. All analyses were made with standard commercial software (IBM SPSS Inc., Armonk, NY, USA; Graph Pad Prism, GraphPad Software, Inc., La Jolla, CA, USA).
Ninety-six patients with SEA were treated within a period of 7 years. Forty-nine patients (51%) underwent early surgery within 12 hours after admission (mean, 6.3 hours) and 47 (49%) delayed surgery later than 12 hours (mean, 51.6 hours). Mean age was 66.2 years (standard deviation [SD], 13.2) in the early surgery group and 65.8 years (SD, 13.1) in the delayed surgery group (p=0.9). The mainly affected region in both groups was the lumbar spine (50 patients; 20 early, 30 delayed, p=0.03) followed by the cervical spine (27 patients; 19 early and 8 late, p=0.02) and the thoracic spine (19 patients; 10 early and 9 late, p=1). The infection affected one level in 62 patients (30 early vs. 32 late; p=0.5), two levels in 19 patients (10 early vs. 9 late, p=0.9) and three or more affected levels in 16 patients (10 early vs. 6 late, p=0.4).
No significant differences were observed regarding prevalence of obesity, time from admission to discharge, diabetes, prior known spinal infection and bony destruction (Table 1).
Surgical intervention included sole decompression of the spinal cord or a nerve root and was performed in 23 patients (16 early vs. 7 late, p=0.05). Single ventral or dorsal instrumentation was performed in 44 patients (25 early vs. 19 late, p=0.3) and 29 patients (8 early vs. 21 late, p=0.002) underwent a combined procedure. Instrumentation was performed in 15 patients within the early surgical procedure and in 18 patients later after initial decompression.
A pathogen causing the infection was identified in the early surgical group in 39 patients (79%) but only in 23 patients (48%) of the delayed surgery group p=0.002. Staphylococcus aureus was the most frequent overall infectious agent (30 early vs. 14 late patients, p=0.002, Table 1).
On admission, patients with neurological deficits (AIS grade A–D) were distributed without significant difference between both groups (73.5% early vs. 70.2% late, p=0.8) as well as patients without neurological deficit (AIS grade E) (26.5% early vs. 29.8% late, p=0.8; Table 2). At last examination before discharge, 38.8% of patients in the early surgery group (38.8% at discharge vs. 73.5% at admission, p=0.001) and 55.3% of patients in the delayed surgery group (55.3% at discharge vs. 70.2% at admission, p=0.2) revealed a persistent neurological deficit (Table 2).
In the early surgery cohort, two patients of the 16 patients with AIS grade A at admission presented with full recovery, six patients improved to AIS grade D, one patient to AIS grade C and two patients to AIS grade B; five patients remained unchanged. One patient classified as AIS grade B deteriorated to grade A, whereas three improved to AIS grade D and three to AIS grade E. Two of three patients with admission grade C improved to AIS grade E while one patient remained unchanged. All patients with initial grade D and E improved to AIS grade E (Table 2).
From 14 patients in the delayed surgery cohort with AIS grade A on admission, nine patients were classified as AIS grade A at discharge, two patients as AIS grade B, one patient as AIS grade C. The other two patients improved to AIS grades D and E, respectively. Four patients were admitted with AIS grade B and underwent surgery more than 12 hours after admission. One of these patients deteriorated to AIS grade A, one patient remained unchanged and the other two improved to AIS grades C and E, respectively. From three patients with AIS grade C on admission two patients presented with a neurological improvement to AIS grades D and E, with one patient remaining unchanged. Patients presenting with AIS grade D on admission remained stable in six cases at discharge, five patients improved to AIS grade E and one patient deteriorated to AIS grade B. One of fourteen patients with the best neurological condition at admission (AIS grade E) deteriorated to grade D, with thirteen patients remaining stable (Table 2).
Mean follow-up was 58 months in the overall study population. The assessment of QOL revealed an overall reduced QOL in patients undergoing delayed surgery with the exception of two items (vitality and social functioning). Significant differences between the early and delayed surgery cohorts were observed in two of four physical items (physical functioning, p=0.009; bodily pain, p=0.04) and in one of four psychological items (role functioning, p=0.04). The highest scores in the early surgery cohort were achieved in physical functioning (86.15), role limitation because of emotional problems (84.61) and social functioning (76.92). The lowest scores were seen in general vitality (60.38), role functioning (61.53) and general health perception (63.30). Outcome based on SF-36 is presented in Fig. 1.
In the abovementioned period 27 patients were identified, which underwent conservative therapy. The mean age in this group was 68.8±14.3 years and 55.6% were males. The infection was located in the cervical spine in four, in the thoracic in nine, and in the lumbar spine in 14 patients. Mean age, sex, the location of infection except cervical infection, number of affected levels, amount of bony destruction at time of diagnosis, the rate of diabetes mellitus among the patients, prior spinal infection did not differ significantly comparing conservatively treated patients with patient undergoing either early or delayed surgery. In this group patients were discharged significantly earlier than surgically treated patients. Obesity was seen significantly often in patients undergoing conservative therapy (p=0.01 compared to patients undergoing early surgery; p=0.04 compared to patients undergoing delayed surgery). Identifying the infectious causing pathogen was an important disadvantage of conservative therapy (22.2% vs. 79.6% and 48.9%). Patients readmitted to hospital as failure of therapy was higher in the conservative treated patients’ populations. Almost 30% of conservatively managed patients were readmitted in the follow up, whereas only 4.1% in the early and 6.4% in the delayed surgery had to undergo further therapy. The reason for readmission was wound healing disorder in the early surgery group, screw displacement in one case and wound infection in two cases as well in the delayed surgery group. Increase of bony destructions was the main reason for hospital readmission in the conservative group. Five patients (18.5%) were treated surgically in the follow up because of failure of conservative therapy.
44.4% of patients undergoing conservative therapy revealed a neurological deficit (AIS A–D) at admission and 55.6% were neurologically intact, which was significantly higher compared to early surgery (55.6% vs. 26.5%, p=0.01) and to the delayed surgery group (55.6% vs. 29.8%, p=0.05) as well (Table 3).
Patients with admission status AIS A remained unchained, whereas one of two patients with admission status AIS grade C remained unchained and one improved to AIS grade D. Eight patients revealed at admission an AIS grade D, four of them remained unchained and four deteriorated to AIS grade C. From 15 patients with AIS grade E on admission, nine patients were classified as AIS E at discharge or follow up, four patients as AIS grade D and two patients as AIS grade C (Table 2).
Comparing all three groups, consisting of early surgery group, delayed surgery group, conservative therapy and similar neurological condition revealed that patients with severe neurological status (AIS A) on admission were significantly often treated surgically (32.7% vs. 29.8% vs. 7.4%). Neurological status according to the AIS grade B, C, and D were almost identical between all three groups. Patients without neurological deficit underwent more often conservative treatment and the proportion was significantly higher in the conservative group. At discharge patients in the conservative treatment group revealed a neurological decline (AIS E, 55.6% vs. 33.3%), whereas patients undergoing early surgery (AIS E, 26.5% vs. 61.2%) and even delayed surgery (AIS E, 29.8% vs. 44.7%) recovered, Tables 3 and 4.
The management of SEA still remains controversial. Despite improvement in surgical techniques, deeper understanding of the pathophysiology and broader availability of medical services, the best timing on evacuation of SEA is still unclear [8-11,24-30]. Several mostly retrospective data previously published favor early surgical treatment. However, the literature on the optimal treatment of SEA displays various limitations: next to the retrospective nature of most publication, another limitation lies within the lack of a consensus of “early” and “late” procedures, as observed in other neurosurgical diseases [13]. Connor et al. [3] reported their series consisting of 77 patients using a 3-day cutoff. In this series, the patients undergoing “early” surgery demonstrated a better neurological improvement without reaching the statistical significant level. Another operative series published by Ghobrial et al. [15] focused on timing of surgery in patients with SAE. The authors did not demonstrate a statistically significant benefit of early surgery (<24 hours after admission) over delayed surgery, as well. Ghobrial and colleagues [15] concluded that surgery within 24 hours seems to be beneficial, with the limitation of not having observed a statistical significance. The importance of early surgery in assurance of a good clinical outcome was assumed by several authors. However, the data justifying this hypothesis could not be provided so far.
The increased accessibility of medical services, early diagnosis and improved surgical techniques guarantee the possibility of early surgery. In this series, surgery within 12 hours after admission could be performed in 49 patients, whereas a high number of patients had to be operated later than 12 hours after admission. Therefore, early surgery could not be performed in these patients. The reason for delayed surgery in symptomatic patients were mostly the delay in diagnosis or poor general condition at admission with subsequent needed therapy to improve the health condition and enabling these patients undergoing surgery. Most patients with spinal infection and epidural abscess were referred from secondary or tertiary hospitals. Here, we could see in some cases a significant delay in establishing the diagnosis.
Most characteristics such as age, sex, diabetes, prior history of surgery, and obesity did not differ significantly in both cohorts. We were able to show that patients undergoing early surgery improved significantly compared to patients undergoing delayed surgery. Another predicting factor of good outcome is the initial clinical status. In the early surgery cohort, all patients with AIS grade D and E improved to AIS grade E. With the exception of two patients with neurological deterioration in the delayed surgery group (from AIS grade E to AIS grade D and AIS grade D to AIS grade B) and six patients all patients improved or remained unchanged in regard to their neurological status. Contrarily, of the patients admitted with poor initial neurological condition in both groups (AIS grade A and B, n=41) only 17 improved to AIS grade D and E.
Nevertheless, the initial neurological status, especially the presence of neurological deficits (AIS grade A–D) did not differ in both groups, as well as predicting factors, such as obesity, diabetes, bony destruction and prior history of spinal infection. Moreover, the clinical course of patients with pure epidural abscesses without osteomyelitis or discitis is certainly different from those with predominant osteomyelitis and may affect the long-term outcome of patients. The distribution of predominant osteomyelitis or discitis was without significant difference (p=0.5) between both groups. Based on these data and comparing almost identical cohorts we now provide evidence, that patients undergoing early surgery reveal a clear benefit regarding neurological status/outcome.
Another benefit of early surgery was the increased rate of identification of the infectious disease-causing pathogen. In the early group and after initial surgery the pathogen was identified almost twice as often when compared to the delayed group. Treatment with antibiotics during an initially nonoperative therapy and/or optimizing the clinical condition for surgery may be the reason for the fact, that pathogens were more often identified in the early surgery group.
To verify our obtained data, which were provided by physicians through physical examination at admission and before discharge, we conducted a prospective collection of data focusing on patient satisfaction undergoing both therapy modalities. The self-reported QOL in both groups was assessed after a mean follow up time of 58 months. Here, we were able to demonstrate that patients undergoing early surgery reveal a better QOL than after delayed evacuation of the SEA. We were therefore able to prove and reevaluate our initial findings after discharge in the follow up period. The beneficial effect of early surgery was reproducible in the follow up period, as well. More specifically, the important outcome parameters physical functioning and bodily pain were significantly better in the early surgery cohort. Role limitation, which is one item of the psychological score was also significantly better in the early surgery cohort, as well. The overall patient satisfaction was better in six of eight items in the early surgery cohort.
One of the important questions in treatment of patients with discitis, osteomyelitis and even with epidural collection of infectious mass remain the effectiveness of conservative therapy. In this study we were able to evaluate the effect of conservative therapy in this patients’ population. Twenty-seven patients underwent conservative therapy with long term antibiotics and immobilization. The basic parameters between all groups, such as age, sex, number of affected spine levels, bony destructions, diabetes, prior spinal infections, predominant discitis/osteomyelitis and rate of died patients in the follow up, did not differ significantly. In conservatively treated patients the infection was mostly located in the lumbar spine. Patients with severe neurological deficits, who had to undergo early surgery revealed often a cervical affection of the spine. Conservatively treated had a shorter hospital stay. In cases of conservative management, patients were mostly sent back to the primary hospital for antibiotic therapy. That means that hospital stay in the authors institution was significantly shorter than patients, who were operated on their spinal infection. The hospital stay in all patients undergoing surgery was essentially longer. The main advantage of surgery, especially of early surgery was the identification of the infectious causing pathogens and initiation of the pathogen specific antibiotic therapy. Here, we could show that the rate of germ-proof in the early surgery group was significantly higher than in the other two groups (79.8% vs. 48.9% vs. 22.2%). The effectiveness of conservative therapy in our investigated patients’ population was not effictive. The number of patients without neurological deficit was significantly higher in the conservative therapy group. Despite adequate therapy we could see an increase of failure of conservative therapy. From 15 patients with AIS grade E at admission only nine remained unchained, whereas the number of patients with neurological deficits (AIS grade A–D) increased over time (44.4% vs. 66.7%).
Another evidence for the inferiority of conservative therapy is the fact that almost 30% of patients were readmitted to our hospital in the follow up and almost 20% underwent subsequent surgery. The reason for the surgery in follow up was a marked increase of the bony destruction with deterioration of the pain situation and occurrence of neurological deficits due to spinal instability.
Moreover, comparing patients without and with evident neurological deficit treated conservatively and surgically revealed that patients undergoing conservative therapy show a decline in neurological status, whereas patients undergoing late surgery reveal an improvement in neurological status but not as effectively as undergoing early surgery, Table 2.
Another concern that could arise by reading the results of the study might be the definition of early surgery, which was defined as 12 hours after admission. In our believe early surgery seems to be beneficial for patients with SEA, the results of our data support the early surgical approach, as well. Nevertheless, until now there is no evidence for any time point performing surgery. Moreover, the effect of conservative treatment is mentioned very rare in the pertaining literature [19,23,27].
Instrumentation and fusion in patients with spinal infection is another often discussed aspect. Inserting of foreign bodies in an infectious situs seems not to be appropriate, whereas many authors published and publish data supporting the beneficial effect of fusion surgery. It provides an adequate surgical debridement and enables immediate stabilization. Surgical treatment with fusion involves debridement of necrotic or infected tissue and irrigation with an antibiotic regimen tailored to treat the responsible pathogen. Furthermore, instrumentation is necessary to bridge the large bony defects and stabilize the spine. Indeed, fusion with graft and internal fixation for refractory pyogenic discitis and vertebral osteomyelitis has been shown to be efficacious in resolving infection, restoring functional mobility, and reducing pain [9,21,22,26].
An important limitation of the present study is that it is a retrospective analysis. It should be considered that most of the surgical cohort comprises patients presenting complications of their infection and/or inadequate response to the conservative therapy. Both groups are difficult to compare, especially because conservative therapy was recommended in cases either presenting with mild manifestation of symptoms or with severe presentation as on the contrary harboring contraindications for surgery. It is to mention that there are some differences affecting the location of infection and severity of the disease. Nevertheless, the aim of this study is to provide an attempt of comparison between the different groups. The Time of surgery was measured from admission to our institution. Actually, it would be better to compare patients from onset of their symptoms than rather from admission. But the onset was very variable in both patients collective and could not evaluated in all cases. In addition, it should be considered that the evaluation of QOL might be influenced by comorbidity and even by social aspects and financial situation. Despite these limitations, patients in the early surgical group revealed a much better QOL. Furthermore, it will be interesting to know how the QOL in these patients was before the manifestation of the spinal infection. A prospective study is necessary to validate the obtained results of our study and to mitigate the occurrence of potential unidentified confounders and selection bias. Hence, over interpretation of the data must carefully be avoided. Since surgeons prefer to perform early surgery on patients with neurological deficits in order to relieve the pressure causing by SEA, we cannot exclude that the inclusion of these patients may elevates the better profile of early surgery.
The results obtained within this study demonstrate a beneficial impact of early surgical treatment of SEA within 12 hours after admission on both the neurological status and the QOL in affected patients. An important prognostic factor of good clinical outcome in our investigated cohort was the initial neurological status. Conservative therapy seems to be inferior to surgical therapy.
Supplementary Materials
The online-only data supplement is available with this article at https://doi.org/10.3340/jkns.2019.0230.
References
1. Adogwa O, Karikari IO, Carr KR, Krucoff M, Ajay D, Fatemi P, et al. Spontaneous spinal epidural abscess in patients 50 years of age and older: a 15-year institutional perspective and review of the literature: clinical article. J Neurosurg Spine. 20:344–349. 2014.

2. Bydon M, De la Garza-Ramos R, Macki M, Naumann M, Sciubba DM, Wolinsky JP, et al. Spinal instrumentation in patients with primary spinal infections does not lead to greater recurrent infection rates: an analysis of 118 cases. World Neurosurg. 82:e807–e814. 2014.

3. Connor DE Jr, Chittiboina P, Caldito G, Nanda A. Comparison of operative and nonoperative management of spinal epidural abscess: a retrospective review of clinical and laboratory predictors of neurological outcome. J Neurosurg Spine. 19:119–127. 2013.

4. Curry WT Jr, Hoh BL, Amin-Hanjani S, Eskandar EN. Spinal epidural abscess: clinical presentation, management, and outcome. Surg Neurol. 63:364–371. discussion 371. 2005.

5. Danner RL, Hartman BJ. Update on spinal epidural abscess: 35 cases and review of the literature. Rev Infect Dis. 9:265–274. 1987.
7. Darouiche RO, Hamill RJ, Greenberg SB, Weathers SW, Musher DM. Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine (Baltimore). 71:369–385. 1992.
8. Davis DP, Wold RM, Patel RJ, Tran AJ, Tokhi RN, Chan TC, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 26:285–291. 2004.

9. Dietz N, Sharma M, Alhourani A, Ugiliweneza B, Wang D, Nuño M, et al. Outcomes of decompression and fusion for treatment of spinal infection. Neurosurg Focus. 46:E7. 2019.

10. Feldenzer JA, McKeever PE, Schaberg DR, Campbell JA, Hoff JT. Experimental spinal epidural abscess: a pathophysiological model in the rabbit. Neurosurgery. 20:859–867. 1987.

11. Foreman SC, Schwaiger BJ, Gempt J, Jungmann PM, Kehl V, Delbridge C, et al. MR and CT Imaging to optimize CT-guided biopsies in suspected spondylodiscitis. World Neurosurg. 99:726–734.e7. 2017.

12. Garrido E, Rosenwasser RH. Experience with the suction-irrigation technique in the management of spinal epidural infection. Neurosurgery. 12:678–679. 1983.

13. Gessler F, Mutlak H, Lamb S, Hartwich M, Adelmann M, Platz J, et al. The impact of tracheostomy timing on clinical outcome and adverse events in poor-grade subarachnoid hemorrhage. Crit Care Med. 43:2429–2438. 2015.

14. Gessler F, Mutlak H, Tizi K, Senft C, Setzer M, Seifert V, et al. Postoperative patient-controlled epidural analgesia in patients with spondylodiscitis and posterior spinal fusion surgery. J Neurosurg Spine. 24:965–970. 2016.

15. Ghobrial GM, Beygi S, Viereck MJ, Maulucci CM, Sharan A, Heller J, et al. Timing in the surgical evacuation of spinal epidural abscesses. Neurosurg Focus. 37:E1. 2014.

16. Hlavin ML, Kaminski HJ, Ross JS, Ganz E. Spinal epidural abscess: a ten-year perspective. Neurosurgery. 27:177–184. 1990.

17. Karikari IO, Powers CJ, Reynolds RM, Mehta AI, Isaacs RE. Management of a spontaneous spinal epidural abscess: a single-center 10-year experience. Neurosurgery. 65:919–923. discussion 923-924. 2009.
18. Khanna RK, Malik GM, Rock JP, Rosenblum ML. Spinal epidural abscess: evaluation of factors influencing outcome. Neurosurgery. 39:958–964. 1996.

19. Kim SD, Melikian R, Ju KL, Zurakowski D, Wood KB, Bono CM, et al. Independent predictors of failure of nonoperative management of spinal epidural abscesses. Spine J. 14:1673–1679. 2014.

20. Koo DW, Townson AF, Dvorak MF, Fisher CG. Spinal epidural abscess: a 5-year case-controlled review of neurologic outcomes after rehabilitation. Arch Phys Med Rehabil. 90:512–516. 2009.

21. Kuklo TR, Potter BK, Bell RS, Moquin RR, Rosner MK. Single-stage treatment of pyogenic spinal infection with titanium mesh cages. J Spinal Disord Tech. 19:376–382. 2006.

22. Lu ML, Niu CC, Tsai TT, Fu TS, Chen LH, Chen WJ. Transforaminal lumbar interbody debridement and fusion for the treatment of infective spondylodiscitis in the lumbar spine. Eur Spine J. 24:555–560. 2015.

23. Lyu RK, Chen CJ, Tang LM, Chen ST. Spinal epidural abscess successfully treated with percutaneous, computed tomography-guided, needle aspiration and parenteral antibiotic therapy: case report and review of the literature. Neurosurgery. 51:509–512. discussion 512. 2002.

24. Özkan N, Wrede K, Ardeshiri A, Hagel V, Dammann P, Ringelstein A, et al. Cervical spondylodiscitis--a clinical analysis of surgically treated patients and review of the literature. Clin Neurol Neurosurg. 117:86–92. 2014.

25. Patel AR, Alton TB, Bransford RJ, Lee MJ, Bellabarba CB, Chapman JR. Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J. 14:326–330. 2014.

26. Przybylski GJ, Sharan AD. Single-stage autogenous bone grafting and internal fixation in the surgical management of pyogenic discitis and vertebral osteomyelitis. J Neurosurg. 94(1 Suppl):1–7. 2001.

27. Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. 23:175–204. discussion 205. 2000.

28. Rigamonti D, Liem L, Sampath P, Knoller N, Namaguchi Y, Schreibman DL, et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. 52:189–196. discussion 197. 1999.

29. Sampath P, Rigamonti D. Spinal epidural abscess: a review of epidemiology, diagnosis, and treatment. J Spinal Disord. 12:89–93. 1999.
30. Savage K, Holtom PD, Zalavras CG. Spinal epidural abscess: early clinical outcome in patients treated medically. Clin Orthop Relat Res. 439:56–60. 2005.
31. Siddiq F, Chowfin A, Tight R, Sahmoun AE, Smego RA Jr. Medical vs surgical management of spinal epidural abscess. Arch Intern Med. 164:2409–2412. 2004.

32. Sinha S, Singh AK, Gupta V, Singh D, Takayasu M, Yoshida J. Surgical management and outcome of tuberculous atlantoaxial dislocation: a 15-year experience. Neurosurgery. 52:331–338. discussion 338-339. 2003.

33. Soehle M, Wallenfang T. Spinal epidural abscesses: clinical manifestations, prognostic factors, and outcomes. Neurosurgery. 51:79–85. discussion 86-87. 2002.

34. Stratton A, Gustafson K, Thomas K, James MT. Incidence and risk factors for failed medical management of spinal epidural abscess: a systematic review and meta-analysis. J Neurosurg Spine. 26:81–89. 2017.

Fig. 1.
Results of patient’s health-related quality of life based on SF-36. PF : physical functioning, RF : role limitation because of physical problems, BP : bodily pain, GH : general health perception, V : vitality, SF : social functioning, RE : role limitation because of emotional problems, MH : general mental health, SF-36 : the Short-Form Health Survey 36.
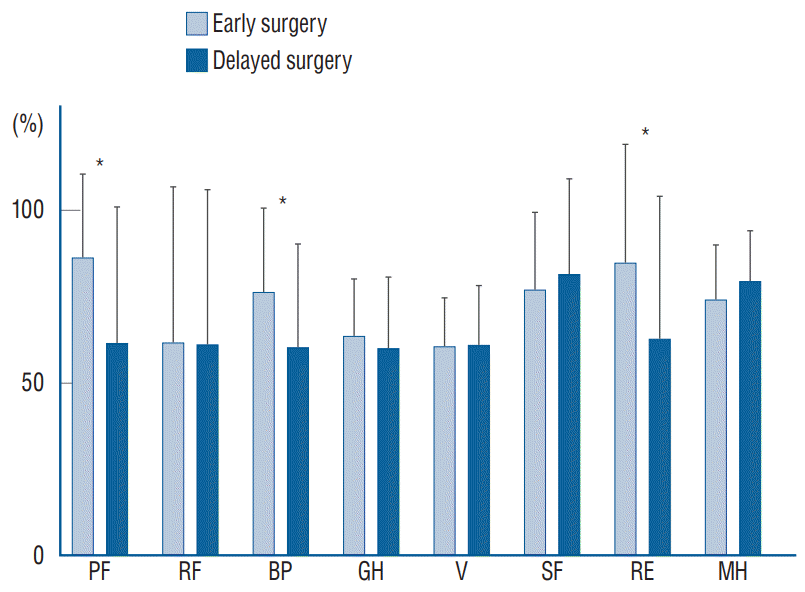
Table 1.
Patient`s characteristics
| Early surgery (n=49) | Delayed surgery (n=47) | Conservative (n=27) | p-value* | p-value† | p-value‡ | |
|---|---|---|---|---|---|---|
| Age (years) | 66.2±13.2 | 65.8±13.1 | 68.8±14.3 | 0.9 | 0.4 | 0.4 |
| Sex, male | 33 (67.3) | 33 (70.2) | 15 (55.6) | 0.8 | 0.3 | 0.2 |
| Location | ||||||
| Cervical | 19 (38.8) | 8 (17.0) | 4 (14.8) | 0.02 | 0.04 | 1 |
| Thoracic | 10 (20.4) | 9 (19.1) | 9 (33.3) | 1 | 0.3 | 0.3 |
| lumbar | 20 (40.8) | 30 (63.8) | 14 (51.9) | 0.03 | 0.5 | 0.3 |
| No. of affected levels | ||||||
| 1 level | 30 (61.2) | 32 (68.1) | 21 (77.8) | 0.5 | 0.2 | 0.4 |
| 2 level | 9 (18.3) | 9 (19.1) | 2 (7.4) | 0.9 | 0.3 | 0.2 |
| 3 and more level | 10 (20.4) | 6 (12.8) | 4 (14.8) | 0.4 | ||
| Surgical technique | ||||||
| Decompression | 16 (32.7) | 7 (14.9) | 0 | 0.05 | 0 | 0 |
| Ventral or dorsal | 25 (51.0) | 19 (40.2) | 0 | 0.3 | 0 | 0 |
| Combined | 8 (16.3) | 21 (44.7) | 0 | 0.003 | 0 | 0 |
| Bony destruction | 8 (16.3) | 15 (31.9) | 9 (33.3) | 0.09 | 0.1 | 1 |
| Hospital stay (days) | 19.1±13.4 | 18.8±13.6 | 10.4±9.8 | 0.9 | 0.004 | 0.006 |
| Identified pathogen | 39 (79.6) | 23 (48.9) | 6 (22.2) | 0.002 | 0.000001 | 0.03 |
| Staphylococcus aureus | 30 (61.2) | 14 (28.8) | 4 (14.8) | 0 | 0 | 0 |
| Miscellaneous | 9 (18.4) | 9 (19.1) | 2 (7.4) | 0 | 0 | 0 |
| Diabetes | 13 (26.5) | 7 (14.9) | 6 (22.2) | 0.2 | 0.8 | 0.5 |
| Obesity | 8 (16.3) | 6 (12.8) | 12 (44.4) | 0.7 | 0.01 | 0.004 |
| Prior spinal infection | 2 (4.1) | 5 (10.6) | 1 (3.7) | 0.2 | 1 | 0.4 |
| Predominant osteomyelitis/discitis | 35 (71.4) | 37 (78.7) | 24 (88.9) | 0.5 | 0.1 | 0.4 |
| Death | 9 (18.4) | 11 (23.4) | 6 (22.2) | 0.6 | 0.8 | 1 |
| Mean time to surgery (hours) | 6.5 | 51.6 | 0 | 0 | 0 | 0 |
| Readmission | 2 (4.1) | 3 (6.4) | 8 (29.6) | 0.7 | 0.002 | 0.01 |
Table 2.
Neurological status on admission and on discharge
Table 3.
Statistical analyze of neurological status on admission and discharge : neurological status on admission
| AIS | Early surgery | Delayed surgery | Conservative therapy | p-value* | p-value† | p-value‡ |
|---|---|---|---|---|---|---|
| A | 16 (32.7) | 14 (29.8) | 2 (7.4) | 0.8 | 0.01 | 0.05 |
| B | 7 (14.3) | 4 (8.5) | 0 | 0.5 | 0 | 0 |
| C | 3 (6.1) | 3 (6.4) | 2 (7.4) | 1 | 1 | 1 |
| D | 10 (20.4) | 12 (25.5) | 8 (29.6) | 0.6 | 0.4 | 0.8 |
| E | 13 (26.5) | 14 (29.8) | 15 (55.6) | 0.8 | 0.01 | 0.05 |
Table 4.
Statistical analyze of neurological status on admission and discharge : neurological status at discharge
| AIS | Early surgery | Delayed surgery | Conservative therapy | p-value* | p-value† | p-value‡ |
|---|---|---|---|---|---|---|
| A | 6 (12.2) | 10 (21.3) | 2 (7.4) | 0.3 | 0.7 | 0.2 |
| B | 2 (4.1) | 4 (8.5) | 0 | 0.4 | 0 | 0 |
| C | 2 (4.1) | 3 (6.4) | 7 (25.9) | 0.7 | 0.008 | 0.03 |
| D | 9 (18.4) | 9 (19.1) | 9 (33.3) | 1 | 0.2 | 0.3 |
| E | 30 (61.2) | 21 (44.7) | 9 (33.3) | 0.2 | 0.03 | 0.5 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download