Abstract
Epilepsy is one of the major chronic neurological diseases affecting many patients. Resection surgery is the most effective therapy for medically intractable epilepsy, but it is not feasible in all patients. Vagus nerve stimulation (VNS) is an adjunctive neuromodulation therapy that was approved in 1997 for the alleviation of seizures; however, efforts to control epilepsy by stimulating the vagus nerve have been studied for over 100 years. Although its exact mechanism is still under investigation, VNS is thought to affect various brain areas. Hence, VNS has a wide indication for various intractable epileptic syndromes and epilepsyrelated comorbidities. Moreover, recent studies have shown anti-inflammatory effects of VNS, and the indication is expanding beyond epilepsy to rheumatoid arthritis, chronic headaches, and depression. VNS yields a more than 50% reduction in seizures in approximately 60% of recipients, with an increase in reduction rates as the follow-up duration increases. The complication rate of VNS is 3–6%, and infection is the most important complication to consider. However, revision surgery was reported to be feasible and safe with appropriate measures. Recently, noninvasive VNS (nVNS) has been introduced, which can be performed transcutaneously without implantation surgery. Although more clinical trials are being conducted, nVNS can reduce the risk of infection and subsequent device failure. In conclusion, VNS has been demonstrated to be beneficial and effective in the treatment of epilepsy and various diseases, and more development is expected in the future.
Epilepsy is a major chronic neurological disease affecting many patients, and approximately 68 million people currently live with epilepsy worldwide [55]. A report in 2013 in Korea showed that the prevalence of epilepsy was 2.28 per 1000 people, which corresponded to approximately 115000 patients nationwide [41]. Medical treatment is the first-line therapy for epilepsy. Nevertheless, 30% of patients with epilepsy remain unaffected by medications [45]. Despite the introduction of several new seizure medications in the past 20 years, the proportion of medically intractable epilepsy cases has not changed much. In a recent longitudinal observational cohort study, the overall 1-year seizure-free rate of newly diagnosed epilepsy patients treated with seizure medication was 64% [15]. For those with medically intractable epilepsy, surgical intervention or a ketogenic diet can be a second-line treatment option.
Vagus nerve stimulation (VNS) is an adjunctive neuromodulation therapy that was approved in 1997 by the United States of America Food and Drug Administration (FDA) as a palliative treatment for patients with intractable focal seizures [73]. The Korean FDA approved VNS in 1999, and the National Health Insurance Service of Korea decided to reimburse the medical cost for patients who were not candidates for curative resection in 2005 [16]. By 2018, VNS devices were implanted into more than 100000 patients worldwide. This is probably not only because VNS yields relatively good clinical outcome but also because it is not a demanding procedure for both patients and neurosurgeons. With innovative devices and wider disease application, the future use of VNS will not be fixed on current indications. This review describes the history, proposed mechanism, clinical outcome, and future implications of VNS as a promising neuromodulation treatment.
Epilepsy has been present in human history, with the first record found in a Babylonian text written 3000 years ago [80]. In the late 18th century, venous hyperemia was suggested as a cause of seizures on the basis of facial flushing and bounding carotid artery pulses during seizures [48]. In the 1880s, New York neurologist James Leonard Corning (1855–1923) developed instruments to decrease cerebral blood f low and electronically stimulated the human vagus nerve to abort seizures [48]. However, contemporaries did not widely accept the use of his instruments due to side effects such as bradycardia, dizziness, and syncope [48].
In the 1900s, animal experiments were continuously carried out to uncover the projections and functions of the vagus nerve. Bailey and Bremer [4] reported an increased amplitude and frequency of the frontal lobe after direct stimulation of the vagus nerves of cats in 1938 [29]. Similarly, in 1949, MacLean et al. stimulated the vagus nerves of monkeys and found inconsistent slow waves from the frontal cortex [29]. In 1951, Dell et al. reported that the stimulation of vagus nerves affected the rhinal sulcus and amygdala in awake cats [29].
Based on these studies, experiments to elucidate the effects and applications of VNS were conducted. Consequently, Zabara stimulated the cervical vagus nerve of dogs with induced seizures and demonstrated an anticonvulsive effect of VNS [29]. Since VNS had emerged as a promising treatment option for epilepsy, Cyberonics Inc. (Houston, TX, USA) was founded and developed a VNS device with a generator, modeled after a cardiac pacemaker in 1987 [81]. By 1988, the first pilot study on VNS implantation in humans for the treatment of epilepsy was carried out in four patients; two patients reported complete seizure control, one reported a 40% seizure frequency reduction, and one reported no effect [59].
Vagus means ‘wandering’ in Latin, and the vagus nerve got its name for its complex course throughout the body. The vagus nerve is the longest cranial nerve in the body and has the most complex functions. The vagus nerve consists of 80% afferent and 20% efferent fibers [25]. The vagus nerve exits the ventrolateral medulla, crosses the subarachnoid space and enters the jugular foramen to the exit cranium. Vagus nerve fibers can be categorized into four categories on the basis of function and tract -specialized visceral efferent fibers, general visceral efferent fibers, somatic afferent fibers, and visceral afferent fibers [12].
Special visceral efferent fibers from the nucleus ambiguus control the muscles of the palate, pharynx and upper esophagus. The recurrent laryngeal nerve controls the intrinsic laryngeal muscle, except the cricothyroid, whereas the superior laryngeal nerve innervates the inferior constrictor and cricothyroid muscles [44]. General visceral efferent fibers arise from the dorsal motor nucleus and synapses in ganglia within their target organs, such as the heart, lungs and digestive tract, providing parasympathetic innervation. Somatic afferent fibers carry sensations from the pharynx, meninges and region around the external auditory meatus. These fibers enter either the jugular (auricular branch, fibers from the meninges of the posterior fossa) or nodose (fibers from the larynx and pharynx) ganglia and then synapse within the spinal trigeminal nucleus [12]. Visceral afferent fibers carry information from chemoreceptors and baroreceptors of the aortic arch, cardiorespiratory system and digestive system and enter nodose ganglion. These afferent fibers are then integrated at the nucleus of the solitary tract (NTS), where various projections to other regions of the brain occur.
The NTS, located in the dorsomedial medulla, is a structure containing a number of neurons that are scattered in the fibrillar plexus [39]. It is a transfer center from the brainstem to the cortex, integrating inputs from the vagus nerve, facial nerve, and glossopharyngeal nerve, and has reciprocal projections to various areas of the central nervous system. These include the hypothalamus, rostral ventrolateral medulla, raphe nuclei, A5 nuclei, area postrema, locus coeruleus, amygdala, thalamus and finally the brain cortex [3]. Because of its numerous projections, in addition to the unidentified functions of various nuclei tracts in the human brain, the exact mechanism of VNS is still under investigation.
One of the early efforts to reveal the mechanism of VNS was to find a change in electroencephalography (EEG) activity. However, EEG did not show significant changes in background activity. In contrast, other studies found increased somatosensory evoked potential inter-peak latency, which suggested that VNS affects more than the vagus nerve [52,53,74].
Cerebral blood f low (CBF) changes were measured and showed various results. Although measurements differed in detail throughout studies, VNS induced measurable changes in CBF along the vagus nerve pathways. One study showed CBF changes in the ipsilateral anterior thalamus and cingulate gyrus using positron emission tomography (PET) with H215O [27]. Another study reported that decreased CBF in the fusiform gyrus correlated with a seizure reduction [13]. In addition, correlations between bilateral thalamic hyperperfusion and decreased seizure frequency were also reported [37].
Neurochemical studies were also performed under the hypothesis that VNS might stimulate the release of neurotransmitters throughout the projection sites of vagus nerves. Pioneering studies first revealed an increase in the metabolites serotonin and dopamine and increased gamma-aminobutyric acid (GABA) and ethanolamine levels in the cerebrospinal fluid of VNS patients [10,34]. It should be noted that the raphe nucleus is a major source of serotonergic neurons, whereas the locus coeruleus and A5 nuclei are sources of noradrenergic neurons, and an increase in brain serotonin levels has an antiepileptic effect [50]. In addition, an increase in GABA transmission in rat NTSs showed a reduction in susceptibility to limbic motor seizures [77]. VNS significantly increased the hippocampal noradrenalin concentration, and a strong positive correlation was found between noradrenergic and anticonvulsive effects [60].
Recently, inflammation has been newly identified as one of the causes of epilepsy [76]. Hence, the role of VNS in the inflammatory pathway has become of interest. Numerous papers have reported the vagus nerve in relation to the immune system, but the effect of VNS in epilepsy has not yet been elucidated [1,7,14,32,49]. Since the vagus nerve has ‘wandering’ and complicated pathways and effects, the exact role of VNS in epilepsy is inconclusive despite its long history of investigation. Future studies are needed.
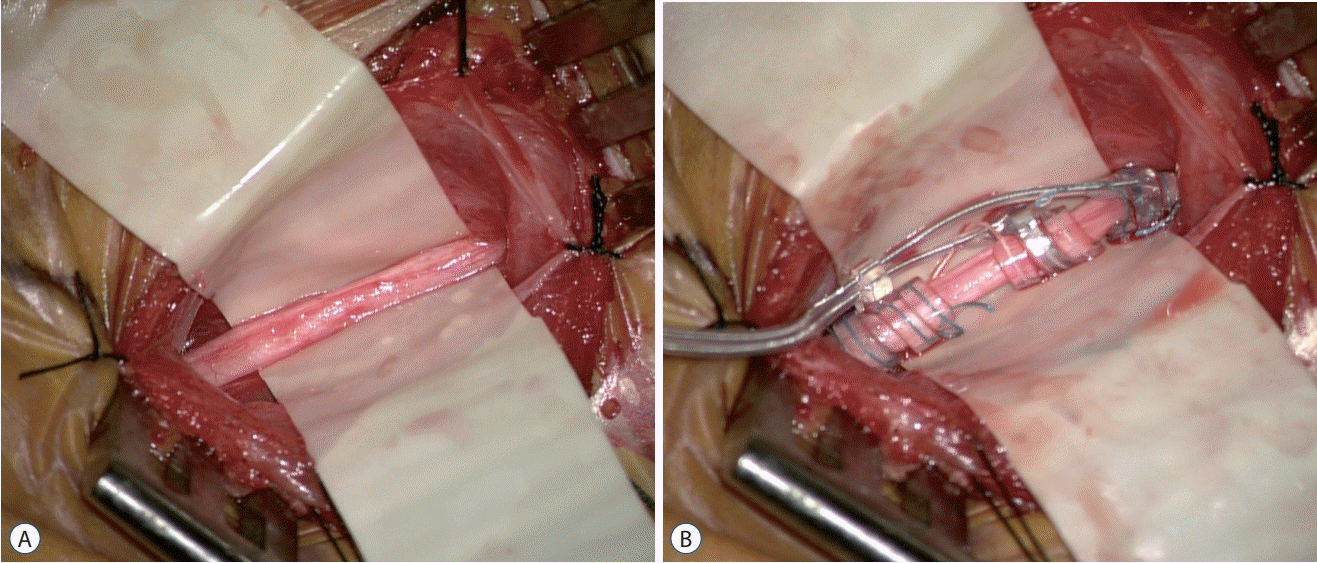
Under general anesthesia, the patient is arranged in a supine position on the operation bed with horse-shoe headrest or donut-shaped pillow. The neck and upper body should be slightly extended and kept higher than the heart to maintain venous drainage. A VNS device is generally implanted on the left side since the right vagus nerve innervates the sinoatrial node, which leads to an increased risk of cardiac complications. The left arm is abducted approximately 80 degrees to expose the lateral aspect of the chest and axilla. After appropriate skin preparation and draping, a 3–4 cm transverse skin incision is made at the skin crease approximately two finger distances above the clavicle between the medial border of the sternocleidomastoid muscle (SCM) and the midline. Blunt dissection along the SCM muscle is made after dividing the platysma fascia until the carotid sheath is identified. Occasionally sensing the pulsation of the carotid artery can help maintain orientation and lowers the risk of unnecessary injury. The vagus nerve can be gently located after opening the carotid sheath. The vagus nerve is usually located slightly behind the common carotid artery and jugular vein, but the configuration can be variable. Using a microscope or surgical loupe is helpful during this procedure. The vagus nerve is carefully dissected from surrounding structures using microforceps to handle the perineurium sheath to avoid direct injury to the vasa nervorum and vagus nerve itself. The vagus nerve is exposed to approximately 4 cm, and the helical coils are wrapped around the vagus nerve (Fig. 1A). Many surgeons start at the tethering (lower) anchor, then progress to the positive (middle) node and the negative (upper) electrode, but it depends on the surgeon’s preference (Fig. 1B). It is important to ensure that the placement of the electrodes is inferior to the cardiac branches of the vagus nerve to avoid cardiac side effects [31]. Two loops should be made from the lead, one at the proximal end and one at the distal end for tension release, and the lead should be anchored to nearest fascia or SCM muscle using head holders. Then, a subcutaneous pocket is created at the left thorax. We preferably make an incision inferomedial to axilla and lateral to the pectoralis muscle for cosmetic reasons. In addition, compared with a subpectoral incision, a para-axillary incision is not associated with a higher infection rate [17]. An approximately 5 cm vertical incision is made, and then blunt dissection through the subcutaneous fat on the suprapectoralis muscle is performed. Subcutaneous tunneling is performed from the thorax to the cervix, the lead and battery is connected, and the battery is placed inside the pocket. Attention is required for children or lean persons who have a thin subcutaneous layer because the device may protrude prominently and cause cosmetic disfigurement or even skin erosion. In our institution, we have lowered such side effects by leaving enough of a fat layer over the generator and by changing the device to a model 103 Demipulse® Generator (Cyberonics Inc., Houston, TX, USA), which is smaller than older models. The battery should be checked before placing to be sure it works properly. An anesthesiologist should be advised when checking the function and prepared for adverse cardiac effects. The battery should also be anchored to the pectoralis muscle. The surgery is completed with closing layer by layer.
The first two single-blinded pilot studies of 14 patients with refractory focal seizures reported mean reductions of 47% and ≥50% in seizure frequency in five patients (36%) after 14 to 35 months of VNS [75]. When the differences between high stimulation and low stimulation were compared, randomized controlled trials showed an approximate 30% reduction in seizure frequency in patients receiving high stimulation (presumed therapeutic dose) and an approximate 15% reduction in seizure frequency in the low stimulation group (presumed subtherapeutic dose) [30,35]. A recent summary of several analyses showed that approximately 60% of patients achieved a ≥50% seizure reduction [56].
VNS therapy is officially approved for patients over the age of 12 years in the USA and many other countries, and the efficacy and safety of VNS has also been proved in children, showing similar outcomes compared to adults. Reports showed a mean reduction of 71% in seizure frequency at the 3-year follow-up (mean age, 11.5 years) and 54% of patients responded with a ≥50% seizure frequency reduction [65,68]. Elliot et al. [21] analyzed 141 children, 61% of whom were under 12 years old, and reported the long-term outcome as a mean reduction rate of 76% 10 years after implantation. Long-term outcomes showed that as the follow-up duration increases, the seizure reduction rate increases correspondingly [21,67].
An analysis of VNS in children under the age of three reported that 33% of patients improved their seizure frequency without significant adverse effects compared to adults. The study also found that negative (normal) magnetic resonance imaging (MRI) was associated with seizure improvement, and VNS reduced the frequency of status epilepticus [24]. Considering the adverse effects, such as cognitive and psychological dysfunction, of prolonged seizure medications initiated at childhood, early VNS for drug refractory epilepsy is a viable option [23,46]. However, protrusion of the device and skin problems may be impediments at very young ages.
VNS is a promising option for pregnant women with medically refractory seizures with no report of teratogenic effects [38]. In addition, a recent study revealed that VNS devices implanted in intractable epilepsy patients resulted in a lower rate of sudden unexpected death in epilepsy (SUDEP) by a rate ratio of 0.68 compared to rates in non-implanted patients [66].
An analysis regarding Asian ethnicity showed no difference. A 4-year follow-up after VNS showed a 60% rate of ≥50% seizure frequency reduction in a Korean study (mean age, 22.3 years; range, 8–44 years) [16]. Chinese colleagues retrospectively analyzed the effects of VNS on children (range, 1–14 years) and showed a 57.2% of patients experienced a ≥50% seizure frequency reduction [78]. A Japanese prospective study revealed a median seizure reduction of 66.2% after 3 years of VNS [42].
A meta-analysis of VNS studies including 74 clinical studies, 3321 patients showed seizure frequency reduction of 51% after 1 year of therapy [22]. The analysis also found that children experience better outcome than adults [22]. Greatest benefit was related to posttraumatic epilepsy with 79% reduction in seizure, and tuberous sclerosis with 68% reduction in seizures [22]. In addition, generalized epilepsy had higher reduction rate than focal seizures, by 58% to 43% [22].
The indications for VNS are wide ranging. VNS showed a favorable outcome for intractable focal and focal-to-bilateral tonic-clonic epilepsy and generalized onset epilepsy, including atonic seizures [47,54,61,64]. Epileptic syndromes such as Lennox-Gastaut syndrome (LGS) and Rett syndrome and epilepsy-related comorbidities such as epileptic encephalopathy, hypothalamic hamartoma, and tuberous sclerosis complex (TSC) have become a good indication for surgery to date [52,57,58,79,82]. A multicenter study of LGS patients revealed a median reduction rate of 57.9% at 6 months [26]. TSC patients showed excellent outcomes with VNS, with nine out of 10 patients presenting at least a 50% reduction [57]. Moreover, six of seven Rett syndrome patients had ≥50% reduction in seizure frequency at 1 year after VNS device implantation [79]. Since VNS affects extensive projections via the NTS, it has a broad spectrum of indications.
VNS has shown various other effects, including improvements in alertness, attention and psychomotor activity [67]. VNS has been approved for treatment-resistant depression patients ≥18 years old with at least one major depressive episode defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [18]. Although VNS has not been developed for use in depression, significant mood improvements were observed, which were independent of effects on seizure activity [19]. Twoyear outcomes of VNS for the treatment of refractory depression showed that 53% of patients had a ≥50% reduction in the Hamilton Rating Scale for Depression (HRSD28) scores from baseline [5]. Similar results were conducted in children who were treated VNS for epilepsy, showing a tendency of improvement in mood scale questionnaire scores [33]. Although more research is needed, VNS could be an effective option for pediatric depression.
Recently, VNS studies for cluster headaches and migraines showed favorable outcomes. Silberstein et al. [69] conducted a randomized double-blind clinical trial of noninvasive VNS in episodic cluster headache patients and found a response rate of 27% in VNS-treated subjects compared to 15% in sham-treated subjects. Similarly, migraine patients with noninvasive VNS achieved pain reduction of 56% at 1 hour and 65% at 2 hours with a ≥50% reduction in visual analog scale (VAS) scores [6]. Since many patients suffering from cluster or migraine headaches complain of unsatisfactory drug response [6,69], stimulating VNS can be helpful.
Notably, VNS has anti-inflammatory properties, and VNS has shown success in treating inflammatory disorders such as rheumatoid arthritis (RA), diabetes, sepsis and cardiovascular diseases [40]. One interesting study revealed that VNS inhibited inf lammatory cytokine, including tumor necrosis factor (TNF) and interleukin (IL)-6, production in RA, as 57% of RA patients with active disease, despite methotrexate therapy, showed a ≥50% improvement [43]. However, the roles of VNS in various psychiatric and functional diseases are still under investigation and development. Additional studies are mandatory to investigate its mechanism of action and application to various diseases.
The vagus nerve is composed of three fibers, A, B, and C, according to conduction velocity. A fibers are myelinated fibers that have the largest diameter and carry visceral afferent and motor input information. B fibers are myelinated fibers with small diameters that carry parasympathetic input information. C fibers are unmyelinated fibers with small diameters that carry visceral afferent information. Fortunately, investigators have found that within human VNS parameters, only A and B fibers are activated due to lower activation thresholds. The activation of C fibers may have unwanted side effects, such as involvement in the cardiopulmonary system, which are not observed in most patients [19].
The most common acute side effects of VNS are infection, vocal cord paresis, and lower facial nerve palsy. Cardiac-related side effects, such as bradycardia and asystole, mainly occurred during device testing but have rarely occurred after initiation [2,72]. Vocal cord paresis and lower facial palsy have become rare due to improved surgical techniques [56]. For longterm use, the most common side effects were stimuli-related cough, throat pain, and hoarseness, all of which tend to improve over time [9]. Compared with adults, children did not show meaningful differences [9].
Since other complications are rare or transient, postoperative infection is the most serious complication to consider. Postoperative infection occurs in 3–6% of patients [9]. Despite some reports suggesting that oral antibiotics are sufficient for treatment [8], many institutions consider surgical removal of all devices mandatory [17,63]. Especially in cases of infection in deep tissue layers, all devices must be removed [20]. This idea fits with our experience in which oral antibiotics were effective only for minor superficial skin infections, and in cases of infections below the skin level, removing devices with intravenous antibiotics was the only effective control for the infection. Studies have reported that revision surgery is feasible and safe [31]. Hence, removing the device should be the first option to consider in cases of overt deep-layer infection.
VNS is generally implanted on the left side. An early VNS experiment was performed in dogs, but the cervical anatomy of humans is much more complicated than that in dogs [62]. In fact, the cervical vagal trunk where the VNS electrodes are normally placed in humans does not have superior or inferior cardiac branches, thereby minimizing clinically relevant cardiac side effects regardless of the side of implantation [44]. There are case reports that right-sided VNS implantation did not show cardiac side effects [51,70]. Thus, right-sided VNS is a considerable option if the left side approach is difficult due to various reasons, such as prior infection on the left side.
Recently, noninvasive VNS (nVNS) has become a new topic. nVNS does not require implantation surgery and stimulation works trans-cutaneously. nVNS involves an intra-auricular electrode (NEMOS, Cerbomed, Erlangen, Germany) or a portable stimulator and digital user interface that controls signal amplitude (gammaCore, electroCore LLC, Basking Ridge, NJ, USA) [11]. NVNS has an advantage compared to conventional VNS as it does not require surgery, minimizing the risk of infection. Stefan et al. [71] stimulated the left auricular branch of the vagus nerve in 10 adult patients, and the seizure frequency was reduced by 45% and 48% in two patients. He et al. [36] also investigated the outcome of transcutaneous VNS and showed a mean reduction seizure frequency of 54% at 6 months. NVNS can be a promising therapy for treating intractable epilepsy in the future when additional clinical trials have been completed. Some adverse effects of nVNS have been reported, such as oropharyngeal pain or neck pain [28].
VNS is an effective neuromodulation that controls intractable epilepsy and has a long history of use. Although the exact mechanism is still under investigation, the outcome of VNS is shown to be favorable with few adverse effects, based on clinical evidence. Furthermore, VNS is applicable to various other diseases, such as depression and chronic pain, brightening the future of neuromodulation therapy.
References
1. Aalbers MW, Klinkenberg S, Rijkers K, Verschuure P, Kessels A, Aldenkamp A, et al. The effects of vagus nerve stimulation on pro- and antiinflammatory cytokines in children with refractory epilepsy: an exploratory study. Neuroimmunomodulation. 19:352–358. 2012.

2. Ali II, Pirzada NA, Kanjwal Y, Wannamaker B, Medhkour A, Koltz MT, et al. Complete heart block with ventricular asystole during left vagus nerve stimulation for epilepsy. Epilepsy Behav. 5:768–771. 2004.

3. Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, et al. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 88:47–75. 1991.

4. Bailey P, Bremer F. A sensory cortical representation of the vagus nerve: with a note on the effects of low blood pressure on the cortical electrogram. J Neurophysiol. 1:405–412. 1938.

5. Bajbouj M, Merkl A, Schlaepfer TE, Frick C, Zobel A, Maier W, et al. Two-year outcome of vagus nerve stimulation in treatment-resistant depression. J Clin Psychopharmacol. 30:273–281. 2010.

6. Barbanti P, Grazzi L, Egeo G, Padovan AM, Liebler E, Bussone G. Noninvasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. J Headache Pain. 16:61. 2015.

7. Barone L, Colicchio G, Policicchio D, Di Clemente F, Di Monaco A, Meglio M, et al. Effect of vagal nerve stimulation on systemic inflammation and cardiac autonomic function in patients with refractory epilepsy. Neuroimmunomodulation. 14:331–336. 2007.

8. Ben-Menachem E. Vagus nerve stimulation, side effects, and long-term safety. J Clin Neurophysiol. 18:415–418. 2001.

9. Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 1:477–482. 2002.

10. Ben-Menachem E, Hamberger A, Hedner T, Hammond EJ, Uthman BM, Slater J, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 20:221–227. 1995.

11. Ben-Menachem E, Revesz D, Simon BJ, Silberstein S. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur J Neurol. 22:1260–1268. 2015.

12. Blumenfeld H, Brainstem I. Surface Anatomy and Cranial Nerves in Blumenfeld H (ed) : Neuroanatomy through Clinical Cases. Sunderland: Sinauer Associates;2010. p. 530–532.
13. Boon P, Vonck K, De Reuck J, Caemaert J. Vagus nerve stimulation for refractory epilepsy. Seizure. 10 Suppl A:456–458. quiz 458-460. 2001.

14. Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 405:458–462. 2000.

15. Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 75:279–286. 2018.

16. Choi SJ, Hong SC, Seo DW, Joo EY, Cho JR, Hwang KJ, et al. Long-term outcome of vagus nerve stimulation for refractory epilepsy: a longitudinal 4 year follow-up study in Korea. J Epilepsy Res. 3:16–20. 2013.

17. Couch JD, Gilman AM, Doyle WK. Long-term expectations of vagus nerve stimulation: a look at battery replacement and revision surgery. Neurosurgery. 78:42–46. 2016.
18. Cristancho P, Cristancho MA, Baltuch GH, Thase ME, O'Reardon JP. Effectiveness and safety of vagus nerve stimulation for severe treatmentresistant major depression in clinical practice after FDA approval: outcomes at 1 year. J Clin Psychiatry. 72:1376–1382. 2011.

19. Elam M. Does vagal nerve stimulation in patients with partial epilepsy affect vagal C-fibers. Neurology. 43:A161–A162. 1993.
20. Elliott RE, Morsi A, Kalhorn SP, Marcus J, Sellin J, Kang M, et al. Vagus nerve stimulation in 436 consecutive patients with treatment-resistant epilepsy: long-term outcomes and predictors of response. Epilepsy Behav. 20:57–63. 2011.

21. Elliott RE, Rodgers SD, Bassani L, Morsi A, Geller EB, Carlson C, et al. Vagus nerve stimulation for children with treatment-resistant epilepsy: a consecutive series of 141 cases. J Neurosurg Pediatr. 7:491–500. 2011.

22. Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg. 115:1248–1255. 2011.

23. Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB. Phenobarbital for febrile seizures--effects on intelligence and on seizure recurrence. N Engl J Med. 322:364–369. 1990.

24. Fernandez L, Gedela S, Tamber M, Sogawa Y. Vagus nerve stimulation in children less than 3 years with medically intractable epilepsy. Epilepsy Res. 112:37–42. 2015.

25. Foley JO, DuBois FS. Quantitative studies of the vagus nerve in the cat. I. The ratio of sensory to motor fibers. J Comp Neurol. 67:49–67. 1937.

26. Frost M, Gates J, Helmers SL, Wheless JW, Levisohn P, Tardo C, et al. Vagus nerve stimulation in children with refractory seizures associated with Lennox-Gastaut syndrome. Epilepsia. 42:1148–1152. 2001.

27. Garnett ES, Nahmias C, Scheffel A, Firnau G, Upton AR. Regional cerebral blood flow in man manipulated by direct vagal stimulation. Pacing Clin Electrophysiol. 15(10 Pt 2):1579–1580. 1992.

28. Gaul C, Diener HC, Silver N, Magis D, Reuter U, Andersson A, et al. Non-invasive vagus nerve stimulation for PREVention and acute treatment of chronic cluster headache (PREVA): a randomised controlled study. Cephalalgia. 36:534–546. 2016.

29. George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM, et al. Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry. 47:287–295. 2000.

30. George R, Sonnen A, Upton A, Salinsky M, Ristanovic R, Bergen D, et al. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology. 45:224–230. 1995.

31. Giordano F, Zicca A, Barba C, Guerrini R, Genitori L. Vagus nerve stimulation: surgical technique of implantation and revision and related morbidity. Epilepsia. 58 Suppl 1:85–90. 2017.

32. Guarini S, Cainazzo MM, Giuliani D, Mioni C, Altavilla D, Marini H, et al. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc Res. 63:357–365. 2004.

33. Hallböök T, Lundgren J, Stjernqvist K, Blennow G, Strömblad LG, Rosén I. Vagus nerve stimulation in 15 children with therapy resistant epilepsy; its impact on cognition, quality of life, behaviour and mood. Seizure. 14:504–513. 2005.

34. Hammond EJ, Uthman BM, Wilder BJ, Ben-Menachem E, Hamberger A, Hedner T, et al. Neurochemical effects of vagus nerve stimulation in humans. Brain Res. 583:300–303. 1992.

35. Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 51:48–55. 1998.

36. He W, Jing X, Wang X, Rong P, Li L, Shi H, et al. Transcutaneous auricular vagus nerve stimulation as a complementary therapy for pediatric epilepsy: a pilot trial. Epilepsy Behav. 28:343–346. 2013.

37. Henry TR. Functional imaging studies of epilepsy therapies. Adv Neurol. 83:305–317. 2000.
38. Houser MV, Hennessy MD, Howard BC. Vagal nerve stimulator use during pregnancy for treatment of refractory seizure disorder. Obstet Gynecol. 115(2 Pt 2):417–419. 2010.

39. Jean A. The nucleus tractus solitarius: neuroanatomic, neurochemical and functional aspects. Arch Int Physiol Biochim Biophys. 99:A3–A52. 1991.
40. Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res. 11:203–213. 2018.

41. Jung J, Seo HY, Kim YA, Oh IH, Lee YH, Yoon SJ. The economic burden of epilepsy in Korea, 2010. J Prev Med Public Health. 46:293–299. 2013.

42. Kawai K, Tanaka T, Baba H, Bunker M, Ikeda A, Inoue Y, et al. Outcome of vagus nerve stimulation for drug-resistant epilepsy: the first three years of a prospective Japanese registry. Epileptic Disord. 19:327–338. 2017.

43. Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A. 113:8284–8289. 2016.

44. Krahl SE. Vagus nerve stimulation for epilepsy: a review of the peripheral mechanisms. Surg Neurol Int. 3(Suppl 1):S47–S52. 2012.

45. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 342:314–319. 2000.

46. Lagae L. Cognitive side effects of anti-epileptic drugs. The relevance in childhood epilepsy. Seizure. 15:235–241. 2006.
47. Landy HJ, Ramsay RE, Slater J, Casiano RR, Morgan R. Vagus nerve stimulation for complex partial seizures: surgical technique, safety, and efficacy. J Neurosurg. 78:26–31. 1993.

48. Lanska DJ. J.L. Corning and vagal nerve stimulation for seizures in the 1880s. Neurology. 58:452–459. 2002.

49. Luyer MD, Greve JW, Hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 202:1023–1029. 2005.

50. Mainardi P, Leonardi A, Albano C. Potentiation of brain serotonin activity may inhibit seizures, especially in drug-resistant epilepsy. Med Hypotheses. 70:876–879. 2008.

51. McGregor A, Wheless J, Baumgartner J, Bettis D. Right-sided vagus nerve stimulation as a treatment for refractory epilepsy in humans. Epilepsia. 46:91–96. 2005.

52. Murphy JV, Wheless JW, Schmoll CM. Left vagal nerve stimulation in six patients with hypothalamic hamartomas. Pediatr Neurol. 23:167–168. 2000.

53. Naritoku DK, Morales A, Pencek TL, Winkler D. Chronic vagus nerve stimulation increases the latency of the thalamocortical somatosensory evoked potential. Pacing Clin Electrophysiol. 15(10 Pt 2):1572–1578. 1992.

54. Nei M, O’connor M, Liporace J, Sperling MR. Refractory generalized seizures: response to corpus callosotomy and vagal nerve stimulation. Epilepsia. 47:115–122. 2006.

55. Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 51:883–890. 2010.

56. Panebianco M, Zavanone C, Dupont S, Restivo DA, Pavone A. Vagus nerve stimulation therapy in partial epilepsy: a review. Acta Neurol Belg. 116:241–248. 2016.

57. Parain D, Penniello MJ, Berquen P, Delangre T, Billard C, Murphy JV. Vagal nerve stimulation in tuberous sclerosis complex patients. Pediatr Neurol. 25:213–216. 2001.

58. Parker AP, Polkey CE, Robinson RO. Vagal nerve stimulation in the epileptic encephalopathies: 3-year follow-up. Pediatrics. 108:221. 2001.

59. Penry JK, Dean JC. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: preliminary results. Epilepsia. 31 Suppl 2:S40–S43. 1990.

60. Raedt R, Clinckers R, Mollet L, Vonck K, El Tahry R, Wyckhuys T, et al. Increased hippocampal noradrenaline is a biomarker for efficacy of vagus nerve stimulation in a limbic seizure model. J Neurochem. 117:461–469. 2011.

61. Ramsay RE, Uthman BM, Augustinsson LE, Upton AR, Naritoku D, Willis J, et al. Vagus nerve stimulation for treatment of partial seizures: 2. safety, side effects, and tolerability. First international vagus nerve stimulation study group. Epilepsia. 35:627–636. 1994.

62. Randall W, et al. Differential innervation of the heart. Cardiac electrophysiology and arrhythmias. 1985.
63. Révész D, Rydenhag B, Ben-Menachem E. Complications and safety of vagus nerve stimulation: 25 years of experience at a single center. J Neurosurg Pediatr. 18:97–104. 2016.

64. Rosenfeld WE, Roberts DW. Tonic and atonic seizures: what’s next--VNS or callosotomy? Epilepsia. 50 Suppl 8:25–30. 2009.

65. Rychlicki F, Zamponi N, Trignani R, Ricciuti RA, Iacoangeli M, Scerrati M. Vagus nerve stimulation: clinical experience in drug-resistant pediatric epileptic patients. Seizure. 15:483–490. 2006.

66. Ryvlin P, So EL, Gordon CM, Hesdorffer DC, Sperling MR, Devinsky O, et al. Long-term surveillance of SUDEP in drug-resistant epilepsy patients treated with VNS therapy. Epilepsia. 59:562–572. 2018.

67. Serdaroglu A, Arhan E, Kurt G, Erdem A, Hirfanoglu T, Aydin K, et al. Long term effect of vagus nerve stimulation in pediatric intractable epilepsy: an extended follow-up. Childs Nerv Syst. 32:641–646. 2016.

68. Shahwan A, Bailey C, Maxiner W, Harvey AS. Vagus nerve stimulation for refractory epilepsy in children: more to VNS than seizure frequency reduction. Epilepsia. 50:1220–1228. 2009.

69. Silberstein SD, Mechtler LL, Kudrow DB, Calhoun AH, McClure C, Saper JR, et al. Non-invasive vagus nerve stimulation for the acute treatment of cluster headache: findings from the randomized, double-blind, shamcontrolled ACT1 study. Headache. 56:1317–1332. 2016.

70. Spuck S, Nowak G, Renneberg A, Tronnier V, Sperner J. Right-sided vagus nerve stimulation in humans: an effective therapy? Epilepsy Res. 82:232–234. 2008.

71. Stefan H, Kreiselmeyer G, Kerling F, Kurzbuch K, Rauch C, Heers M, et al. Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: a proof of concept trial. Epilepsia. 53:e115–e118. 2012.

72. Tatum WO 4th, Moore DB, Stecker MM, Baltuch GH, French JA, Ferreira JA, et al. Ventricular asystole during vagus nerve stimulation for epilepsy in humans. Neurology. 52:1267–1269. 1999.

73. Terry Jr RS. Vagus nerve stimulation therapy for epilepsy in Holmes MD (ed) : Epilepsy Topics. London: Intech;2014. p. 139–160.
74. Tougas G, Hudoba P, Fitzpatrick D, Hunt RH, Upton AR. Cerebralevoked potential responses following direct vagal and esophageal electrical stimulation in humans. Am J Physiol. 264(3 Pt 1):G486–G491. 1993.

75. Uthman BM, Wilder BJ, Penry JK, Dean C, Ramsay RE, Reid SA, et al. Treatment of epilepsy by stimulation of the vagus nerve. Neurology. 43:1338–1345. 1993.

76. Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 7:31–40. 2010.

77. Walker BR, Easton A, Gale K. Regulation of limbic motor seizures by GABA and glutamate transmission in nucleus tractus solitarius. Epilepsia. 40:1051–1057. 1999.

78. Wang X, Gao Q, Zhou J, Guan Y, Liu C, Pan W, et al. The neuropsychological efficacy of vagus nerve stimulation in 56 children with catastrophic epilepsy. Neuropsychiatry. 7:387–392. 2017.

79. Wilfong AA, Schultz RJ. Vagus nerve stimulation for treatment of epilepsy in Rett syndrome. Dev Med Child Neurol. 48:683–686. 2006.

80. World Health Organization. Atlas: Epilepsy Care in the World, 2005. Geneva: World Health Organization;2005. p. 16.
81. Wright CW, Bu L, Jones A, Green NC. VNS therapy for the treatment of epilepsy in Majid A (ed) : Electroceuticals. New York: Springer;2017. p. 181–204.
Fig. 1.
A : Vagus nerve is exposed on a rubber sheet. The carotid sheath is opened and tied to adjacent soft tissue. The exposure should be at least 4 cm to properly locate the helical electrode. B : A helical electrode is successfully installed. Helices are composed of tethering anchor, positive and negative electrodes in order from the bottom, respectively.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download