Abstract
Objective
Methods
Results
Conclusion
References
Fig. 1.
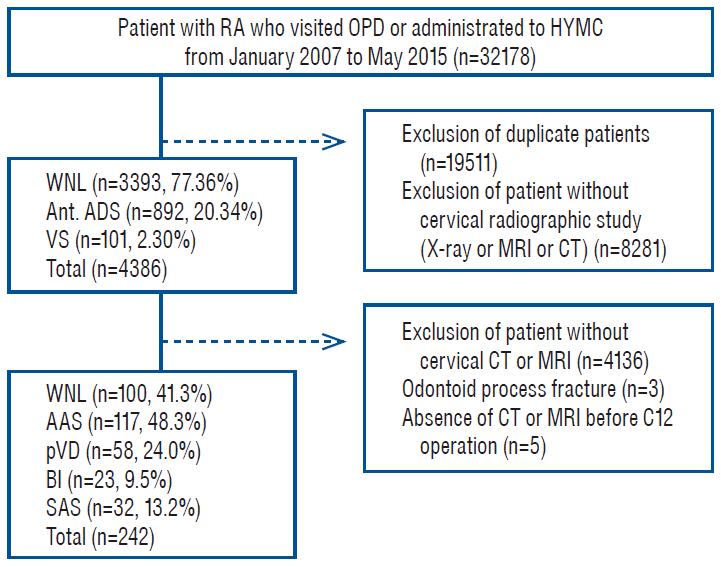
Fig. 2.
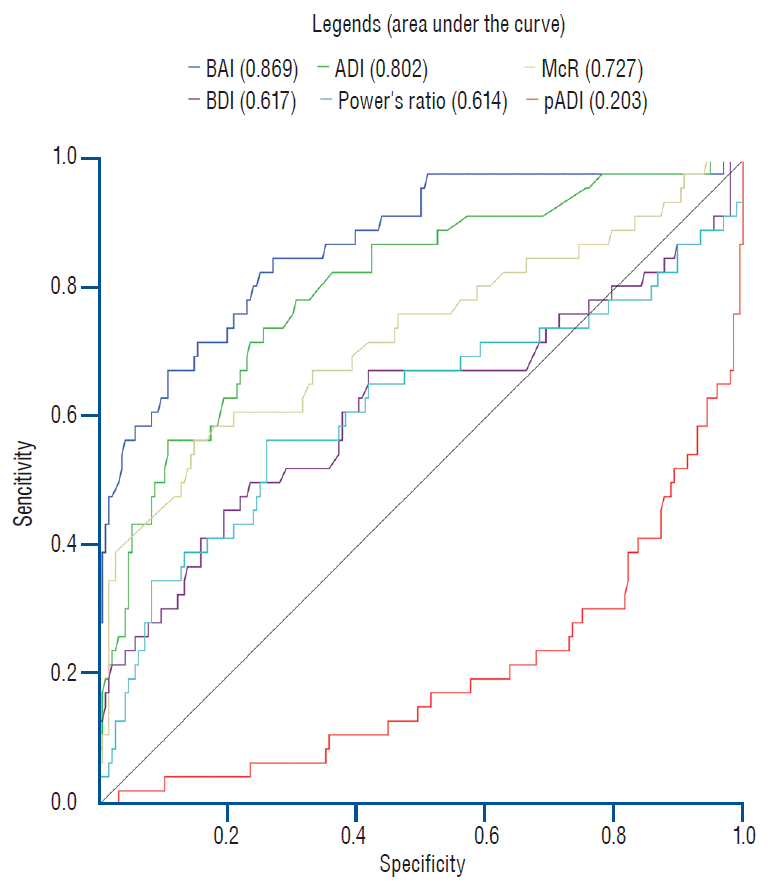
Table 1.
| Normal | VD | BI | p-value (CC) | |
|---|---|---|---|---|
| Total patients | 161 (66.5) | 58 (24.0) | 23 (9.5) | |
| Sex (female) | 131 (81.4) | 53 (91.4) | 22 (95.7) | 0.018 (0.152) |
| Age (years) | 56.14±12.81 | 57.31±11.45 | 58.74±12.70 | 0.422 (0.052) |
| ADI | 2.79±1.81 | 3.36±2.21 | 4.67±3.02 | 0.004 (0.183) |
| PADI | 16.42±3.09 | 15.66±2.87 | 14.69±2.66 | 0.008 (-0.170) |
| Power's ratio | 0.70±0.07 | 0.70±0.77 | 0.75±0.10 | 0.041 (0.132) |
| McR | -5.40±1.48 | -1.35±1.14 | 3.70±2.33 | 0.000 (0.832) |
| BDI | 6.66±1.94 | 3.82±2.27 | 4.80±1.76 | 0.000 (-0.462) |
| BAI | 7.47±4.46 | 8.55±3.44 | 12.25±4.09 | 0.000 (0.321) |
| Bony erosion | 54 (33.5) | 33 (43.1) | 30 (87.0) | 0.000 (0.327) |
| Pannus formation | 39 (24.2) | 16 (27.6) | 6 (26.1) | 0.645 (0.030) |
| CMJ compression | 18 (11.2) | 10 (17.2) | 18 (78.3) | 0.000 (0.358) |
| C12 fixation | 20 (12.4) | 15 (25.9) | 10 (43.5) | 0.000 (0.241) |
| AAS | 61 (62.1) | 37 (63.8) | 19 (82.6) | 0.000 (0.308) |
| SAS | 13 (8.1) | 9 (15.5) | 11 (47.8) | 0.000 (0.269) |
| Wrist Jt. erosion (Uni/Bi) | 18/48 (12.5/33.3) | 8/33 (15.7/64.7) | 0/20 (0/90.9) | 0.000 (0.402) |
| Finger deformity | 28 (19.4) | 22 (42.3) | 13 (59.1) | 0.000 (0.304) |
| Steroid | 128 (20.5) | 46 (20.7) | 20 (87.0) | 0.619 (0.032) |
| DMARDs | 120 (74.5) | 50 (86.2) | 22 (95.7) | 0.006 (0.176) |
| Immunosupressants | 126 (78.3) | 47 (81.0) | 20 (87.0) | 0.365 (0.058) |
| Biologic drugs | 28 (17.4) | 9 (15.5) | 4 (17.4) | 0.826 (-0.014) |
| ESR* | 86 (54.1) | 47 (81) | 18 (78.3) | 0.000 (0.249) |
| CRP* | 80 (50.3) | 41 (70.7) | 18 (78.3) | 0.000 (0.219) |
| Anti-CCP antibody* | 89 (84.8) | 30 (75.0) | 12 (100) | 0.781 (-0.022) |
| RF* | 108 (83.7) | 42 (80.8) | 15 (75) | 0.369 (-0.064) |
VS : vertical subluxation, VD : vertical dislocation, BI : basilar invagination, CC : correlation coefficient, ADI : atlantodental interval, PADI : posterior-atlantodental interval, McR : McRae’s line and the tip of odontoid process of the axis, BDI : basin-dental interval, BAI : basin-posterior axial line interval, CMJ : cervicomedullary junction, AAS : atlantoaxial subluxation, SAS : subaxial subluxation, Jt. : joint, Uni : unilateral, Bi : bilateral, DMARDs : disease-modifying antirheumatic drugs, ESR : erythrocyte sedimentation rate, CRP : c-reactive protein, CCP : cyclic citrullinated peptide, RF : rheumatoid factor
Table 2.
| Normal | AAS | p-value (CC) | |
|---|---|---|---|
| Total patients | 125 (51.7) | 117 (48.3) | |
| Sex (female) | 100 (80) | 106 (90.6) | 0.021 (0.149) |
| Age (years) | 56.73±12.28 | 56.60±12.71 | 0.912 (0.007) |
| ADI | 1.70±0.55 | 4.61±2.14 | 0.000 (0.731) |
| PADI | 17.16±2.38 | 24.92±3.24 | 0.000 (-0.398) |
| Power's ratio | 0.70±0.07 | 0.71±0.08 | 0.281 (0.070) |
| McR | -4.55±2.70 | -2.51±3.51 | 0.000 (0.317) |
| BDI | 5.73±2.11 | 5.88±2.97 | 0.779 (0.018) |
| BAI | 6.11±2.42 | 10.39±4.94 | 0.000 (0.578) |
| Bony erosion | 31 (24.8) | 76 (65.0) | 0.000 (0.404) |
| Pannus formation | 20 (16.0) | 41 (35.0) | 0.001 (0.219) |
| CMJ compression | 3 (2.4) | 43 (36.8) | 0.000 (0.438) |
| C12 fixation | 9 (7.2) | 36 (30.8) | 0.000 (0.303) |
| SAS | 12 (9.6) | 21 (17.9) | 0.084 (0.112) |
| Steroid | 102 (81.6) | 92 (78.6) | 0.565 (-0.037) |
| DMARDs | 99 (79.2) | 93 (79.5) | 0.956 (0.004) |
| Immunosupressants | 92 (73.6) | 101 (86.3) | 0.004 (0.249) |
| Biologic drugs | 21 (16.8) | 20 (17.1) | 0.952 (0.004) |
| Wrist Jt. erosion (Uni/Bi) | 13/33 (10.4/26.4) | 13/68 (11.1/58.1) | 0.000 (0.412) |
| Finger deformity | 20 (17.4) | 43 (41.7) | 0.000 (0.268) |
| ESR* | 69 (56.1) | 82 (70.1) | 0.209 (0.145) |
| CRP* | 67 (54.0) | 72 (62.1) | 0.025 (0.081) |
| Anti-CCP antibody* | 66 (83.5) | 65 (83.3) | 0.972 (-0.003) |
| RF* | 83 (83.0) | 82 (81.2) | 0.739 (-0.024) |
AAS : atlantoaxial subluxation, CC : correlation coefficient, ADI : atlantodental interval, PADI : posterior-atlantodental interval, McR : McRae’s line and the tip of odontoid process of the axis, BDI : basin-dental interval, BAI : basin-posterior axial line interval, CMJ : cervicomedullary junction, SAS : subaxial subluxation, DMARDs : disease-modifying antirheumatic drugs, Jt. : joint, Uni : unilateral, Bi : bilateral, ESR : erythrocyte sedimentation rate, CRP : c-reactive protein, CCP : cyclic citrullinated peptide, RF : rheumatoid factor
Table 3.
| Normal | CMJ compression | p-value (CC) | |
|---|---|---|---|
| Total patients | 196 (81.0) | 46 (19.0) | |
| Sex (female) | 163 (83.2) | 43 (93.5) | 0.077 (0.144) |
| Age (years) | 56.25±12.82 | 58.43±10.78 | 0.348 (0.061) |
| ADI | 2.62±1.67 | 5.17±2.54 | 0.000 (0.411) |
| PADI | 16.69±2.73 | 13.47±2.94 | 0.000 (-0.404) |
| Power's ratio | 0.70±0.07 | 0.73±0.10 | 0.015 (0.156) |
| McR | -4.21 (2.55) | -0.80 (4.42) | 0.000 (0.309) |
| BDI | 5.56±2.23 | 6.80±3.50 | 0.013 (0.159) |
| BAI | 7.04±2.82 | 13.03±6.27 | 0.000 (0.502) |
| Bony erosion | 72 (36.7) | 35 (76.1) | 0.000 (0.311) |
| Pannus formation | 40 (20.4) | 21 (45.7) | 0.000 (0.228) |
| C12 fixation | 28 (14.3) | 17 (37.0) | 0.000 (0.229) |
| BI | 5 (2.6) | 18 (39.1) | 0.000 (0.489) |
| AAS | 74 (37.8) | 43 (93.5) | 0.000 (0.438) |
| SAS | 18 (9.2) | 15 (32.6) | 0.000 (0.268) |
| Wrist Jt. Erosion (Uni/Bi) | 12/69 (12.1/39.9) | 5/22 (11.4/72.7) | 0.000 (0.279) |
| finger deformity | 45 (25.9) | 18 (40.9) | 0.049 (0.133) |
| Steroid | 158 (80.6) | 36 (78.3) | 0.720 (-0.023) |
| DMARDs | 154 (78.6) | 38 (82.6) | 0.545 (0.039) |
| Immunosuppressants | 155 (79.1) | 38 (82.6) | 0.594 (0.034) |
| Biologic drugs | 33 (16.8) | 8 (17.4) | 0.928 (0.006) |
| ESR* | 118 (60.8) | 33 (71.7) | 0.170 (0.089) |
| CRP* | 107 (55.2) | 32 (69.6) | 0.076 (0.115) |
| Anti-CCP antibody* | 103 (81.1) | 28 (93.3) | 0.106 (0.129) |
| RF* | 129 (80.1) | 36 (90.0) | 0.146 (0.103) |
CMJ : cervicomedullary junction, CC : correlation coefficient, ADI : atlantodental interval, PADI : posterior-atlantodental interval, McR : McRae’s line and the tip of odontoid process of the axis, BDI : basin-dental interval, BAI : basin-posterior axial line interval, BI : basilar invagination, AAS : atlantoaxial subluxation, SAS : subaxial subluxation, Jt. : joint, Uni : unilateral, Bi : bilateral, DMARDs : disease-modifying antirheumatic drugs, ESR : erythrocyte sedimentation rate, CRP : c-reactive protein, CCP : cyclic citrullinated peptide, RF : rheumatoid factor




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download