Abstract
Objective
Methods
Results
Notes
FUNDING
This work was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2015R1A3A2032927 to W.U.K. and 2018R1D1A1B07045491 to J.H.K.). This study was supported by Research Fund of Seoul St. Mary’s Hospital, The Catholic University of Korea.
AUTHOR CONTRIBUTIONS
Y.P. and W.U.K. conceived and planned the study. Y.P., J.W.K., J.H.K., and W.U.K. recruited the subjects, and collected clinical information and blood samples. Y.P. and M.L.L. conducted the experiments using the blood samples. Y.P., Y.J.P., and W.U.K. analyzed the data. Y.P. and W.U.K. drafted and revised the manuscript. All authors provided critical feedback and helped with research, analyses, and manuscript writing.
REFERENCES
Fig. 1
Fig. 2
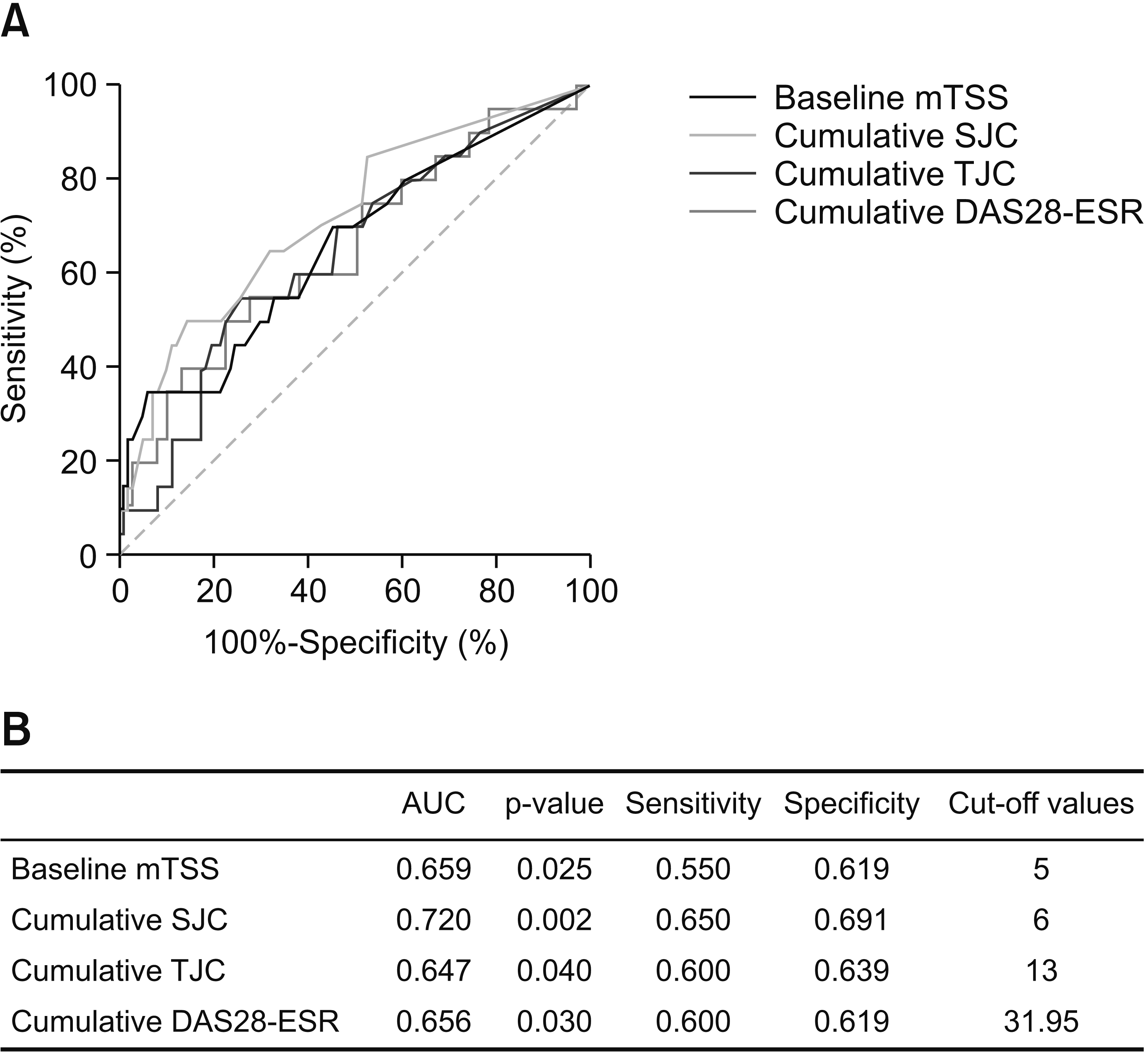
Table 1
| Variable | Total (n=117) | RP (+) (n=20) | RP (−) (n=97) | p-value |
|---|---|---|---|---|
| Age (yr) | 57 (49~65) | 55 (46~63) | 57 (49~66) | 0.411 |
| Female | 102 (87.2) | 17 (85.0) | 85 (87.6) | 0.720 |
| Body mass index (kg/m2) | 22.8 (20.6~24.9) | 22.4 (20.7~24.3) | 22.9 (20.6~25.0) | 0.728 |
| Disease duration (yr) | 6 (1~13) | 7 (3~14) | 5 (1~13) | 0.606 |
| X-ray f/u duration (mo) | 15 (13~19) | 16 (13~23) | 15 (13~19) | 0.588 |
| Smoking | 1 (0.9) | 1 (5.0) | 0 (0) | 0.171 |
| Medication status | ||||
| Glucocorticoid use | 88 (75.2) | 13 (65.0) | 75 (77.3) | 0.263 |
| Pd equivalent (mg) | 5.0 (2.5~5.0) | 5.0 (2.5~7.5) | 5.0 (2.5~5.0) | 0.378 |
| csDMARDs | ||||
| Methotrexate | 73 (62.4) | 13 (65.0) | 60 (61.9) | 0.792 |
| Leflunomide | 55 (47.0) | 11 (55.0) | 44 (45.4) | 0.432 |
| Hydroxychloroquine | 62 (53.0) | 12 (60.0) | 50 (51.5) | 0.490 |
| Sulfasalazine | 7 (6.0) | 0 (0) | 7 (7.2) | 0.602 |
| bDMARDs or tsDMARDs | 55 (47.0) | 11 (55.0) | 44 (45.4) | 0.432 |
| Tocilizumab | 25 | 6 | 19 | |
| Abatacept | 18 | 3 | 15 | |
| Etanercept | 4 | 0 | 4 | |
| Adalimumab | 3 | 0 | 3 | |
| Tofacitinib | 3 | 2 | 1 | |
| Baricitinib | 2 | 0 | 2 | |
| DAS28 status* | ||||
| Remission | 49 (41.9) | 6 (20.0) | 43 (44.3) | |
| LDA | 17 (14.5) | 3 (15.0) | 13 (14.4) | |
| MDA | 32 (27.4) | 6 (30.0) | 26 (26.8) | |
| HDA | 19 (16.2) | 4 (20.0) | 15 (15.5) | |
| MDA or HDA | 51 (43.6) | 10 (50.0) | 41 (42.3) | 0.525 |
| DAS28 change† | ||||
| Stable | 72 (61.5) | 14 (70.0) | 58 (59.8) | |
| Improvement | 38 (32.5) | 5 (25.0) | 33 (34.0) | |
| Deterioration | 7 (6.0) | 1 (5.0) | 6 (6.2) | |
| Target achievement‡ | 96 (82.1) | 14 (70.0) | 82 (84.5) | 0.196 |
Values are presented as median (interquartile range) or number (%). RP: radiographic progression, f/u: follow up, Pd: prednisolone, csDMARDs: conventional synthetic disease modifying anti-rheumatic drugs, bDMARDs: biologic disease modifying anti-rheumatic drugs, tsDMARDs: targeted synthetic disease modifying anti-rheumatic drugs, DAS28: disease activity score with 28 joint counts, LDA: low disease activity, MDA: moderate disease activity, HDA: high disease activity, ESR: erythrocyte sedimentation rate. *DAS28 status was evaluated at baseline and remission, LDA, MDA, and HDA were defined according to DAS28-ESR. †DAS28 changes defined as ‘Stable’ indicate no interval change in the DAS28 during the study period. ‘Improvement’ denotes a change from MDA or HDA to remission or LDA during the study periods. ‘Deterioration’ denotes a change from remission or LDA to MDA or HDA. ‡Target achievement is defined as achievement of remission or LDA at the last visit.
Table 2
| Variable | Total (n=117) | RP (+) (n=20) | RP (–) (n=97) | p-value |
|---|---|---|---|---|
| Tender joint count | 1 (0~4) | 1 (0~4) | 1 (0~3) | 0.362 |
| Swollen joint count | 0 (0~2) | 1 (0~4) | 0 (0~2) | 0.101 |
| Visual analog scale | 50 (30~70) | 45 (35~68) | 50 (30~70) | 0.785 |
| DAS28-ESR | 2.94 (1.80~4.65) | 3.20 (1.93~4.68) | 2.90 (1.75~4.65) | 0.480 |
| Baseline mTSS | 3 (0~18) | 8 (1~52) | 2 (0~16) | 0.022 |
| WBC count (×103/mm3) | 6.48 (5.08~7.64) | 6.61 (4.34~8.01) | 6.48 (5.45~7.52) | 0.534 |
| Hemoglobin (g/dL) | 13.0 (12.2~13.7) | 13.1 (12.5~14.0) | 13.0 (12.1~13.6) | 0.467 |
| Platelet count (×103/mm3) | 256 (207~315) | 276 (222~334) | 254 (206~315) | 0.259 |
| Albumin (g/dL) | 4.1 (3.9~4.4) | 4.1 (3.9~4.4) | 4.2 (3.9~4.4) | 0.788 |
| RF (IU/mL) | 76.7 (25.7~182.0) | 48.9 (18.7~461.9) | 77.8 (27.1~171.4) | 0.775 |
| RF positivity | 93 (79.5) | 15 (75.0) | 78 (80.4) | 0.556 |
| RF high positivity* | 66 (56.4) | 9 (45.0) | 57 (58.8) | 0.258 |
| ACPA positivity | 31/37 (83.8) | 5/6 (83.3) | 26/31 (83.9) | 0.999 |
| ACPA high positivity* | 29/37 (78.4) | 5/6 (83.3) | 24/31 (77.4) | 0.999 |
| ESR (mm/h) | 14 (3~31) | 19 (3~48) | 12 (3~31) | 0.561 |
| CRP (mg/dL) | 0.19 (0.04~1.12) | 0.16 (0.04~2.22) | 0.21 (0.04~1.07) | 0.828 |
| sCD14 (pg/mL) | 1,961 (1,725~2,392) | 2,071 (1,833~2,300) | 1,922 (1,714~2,431) | 0.588 |
Values are presented as median (interquartile range) or number (%). RP: radiographic progression, DAS28: Disease Activity Score 28, ESR: erythrocyte sedimentation rate, mTSS: modified total Sharp score, WBC: white blood cell, RF: rheumatoid factor, ACPA: anti-citrullinated protein antibody, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, sCD14: soluble CD14. *High positivity is defined as >3× the upper limit of normal.
Table 3
| Variable | Uni-variable (not adjusted) | Model 1* (uni-variable) | Model 2† (multi-variable) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p-value | Odds ratio | 95% CI | p-value | Odds ratio | 95% CI | p-value | |||
| Baseline mTSS | 1.024 | 1.007~1.041 | 0.022 | 1.036 | 1.011~1.062 | 0.004 | 1.036 | 1.010~1.063 | 0.006 | ||
| Cumulative RF (IU/mL) | 1.000 | 1.000~1.000 | 0.854 | 1.000 | 1.000~1.000 | 0.102 | |||||
| Cumulative ESR (mm/h) | 1.003 | 1.000~1.006 | 0.134 | 1.004 | 1.001~1.007 | 0.013 | 1.001 | 0.993~1.008 | 0.887 | ||
| Cumulative CRP (mg/dL) | 1.021 | 0.990~1.053 | 0.134 | 1.024 | 0.993~1.057 | 0.133 | |||||
| Cumulative TJC | 1.020 | 1.001~1.040 | 0.037 | 1.026 | 1.004~1.048 | 0.021 | 0.978 | 0.936~1.014 | 0.384 | ||
| Cumulative SJC | 1.090 | 1.029~1.155 | 0.001 | 1.099 | 1.034~1.167 | 0.002 | 1.099 | 1.019~1.185 | 0.014 | ||
| Cumulative DAS28-ESR | 1.053 | 1.013~1.095 | 0.029 | 1.060 | 1.018~1.103 | 0.005 | 1.017 | 0.894~1.156 | 0.802 | ||
| Cumulative sCD14 (pg/mL) | 1.000 | 1.000~1.000 | 0.148 | 1.000 | 1.000~1.000 | 0.245 | |||||
CI: confidence interval, mTSS: modified total Sharp score, RF: rheumatoid factor, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, TJC: tender joint count, SJC: swollen joint count, DAS28: disease activity score 28, sCD14: soluble CD14. *Model 1: adjusted for age, sex, and disease duration. †Model 2: clinical variables with p-values < 0.1 in ‘Model 1’ were included in multi-variable analysis with adjustment for age, sex, and disease duration.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download