Abstract
Long QT syndrome is a cardiac repolarization disorder and is associated with an increased risk of torsades de pointes. The acquired form is most often attributable to administration of specific medications and/or electrolyte imbalance. This review provides insights into the risk for QT prolongation associated with drugs frequently used in the treatment of chronic pain. In the field of pain medicine all the major drug classes (i.e. NSAIDs, opioids, anticonvulsive and antidepressant drugs, cannabinoids, muscle relaxants) contain agents that increase the risk of QT prolongation. Other substances, not used in the treatment of pain, such as proton pump inhibitors, antiemetics, and diuretics are also associated with long QT syndrome. When the possible benefits of therapy outweigh the associated risks, slow dose titration and electrocardiography monitoring are recommended.
Long QT syndrome is a cardiac repolarization disorder, and is associated with an increased risk of torsades de pointes (TdP), a life-threatening type of polymorphic ventricular tachycardia, and sudden cardiac death [1]. Acquired and congenital forms can be distinguished. Acquired long QT syndrome is most often attributable to administration of specific medications and/or electrolyte imbalance.
Polypharmacy is frequently encountered in patients suffering from chronic pain. The coexistence of pain and depression is common, as is co-treatment for these conditions [2]. The association of antidepressants and antipsychotic medications with prolongation of the QT interval is well known [3]. However, many clinicians are less aware of the possible arrhythmic potential of other types of medication used to treat pain. This review provides insights into the risk for QT prolongation associated with drugs frequently used in the treatment of chronic pain.
The QT interval is defined as the duration from the beginning of the QRS complex to the end of the T wave. It is a surrogate parameter of ventricular depolarization and repolarization in the surface electrocardiogram (ECG). Heart rate influences the QT duration, so it is common to present the rate-corrected QT interval (QTc). This is calculated from Bazett's formula:
Prolongation of the QT interval above 470 ms for men and 480 ms for women should be regarded as abnormal [4]. Several risk factors for QT prolongation have been identified, including female sex, advanced age, drug-drug interactions, genetic predisposition, hypokalemia, hypomagnesemia, heart failure, and bradycardia [56]. To reduce the risk of developing TdP, the American Heart Association/American College of Cardiology recommends ECG recordings before and 8–12 hours after an initiation or increase in doses of medication associated with an increased risk of QT prolongation [4]. In the pathomechanism of the acquired long QT syndrome, the human ether-a-go-go related gene (hERG), a voltage gated potassium channel, plays a pivotal role, as it is blocked by various medications [7].
The use of non-steroidal anti-inflammatory drugs (NSAIDs), whether prescribed or self-administered, is extremely prevalent. A recent epidemiologic study reported that nearly one third of the general population had used NSAIDs within the previous 4 weeks [8]. The cardiovascular risks associated with NSAIDs are well known and commonly attributed to thrombotic events caused by the inhibitory effect of cyclooxygenases inhibition on platelets and the endothelium; however, alteration of cardiac repolarization is a completely different mechanism which also increases cardiac non-thrombotic risk [9].
Ketorolac has been confirmed to increase QT duration. In one study, a single dose of ketorolac increased the QT duration by > 30 msec. In patients undergoing general anesthesia, a single dose of ketorolac increased QT duration by 58% [10].
Pathak et al. [11] presented three patients developing TdP within days after initiation of celecoxib therapy. Two of them had a preexisting history of long QT syndrome. In an ex-vivo experiment, celecoxib inhibited hERG channels [12].
For diclofenac, an in-vitro study observed no prolongation of repolarization in therapeutic doses. However, in the case of reduced repolarization reserves, high doses of diclofenac may lengthen repolarization and enhance pro-arrhythmic risk [13].
Methadone is well known to increase the QT duration in a dose-dependent manner [14]. A recent prospective analysis of initiation of methadone therapy (maximum daily dose = 60 mg) in patients with chronic pain has found that 11% had a QTc > 450 ms at any time point and that the highest incidence was observed after 1 month of treatment [15]. Similar incidences of long QT syndrome have been reported in other studies investigating pain patients on stabile methadone doses [16].
In its clinical practice guideline on the safety of methadone, the American Pain Society emphasizes the risk of QT prolongation when using this agent [17]. ECG changes consistent with blockade of sodium and potassium channels, including long QT, are described for tramadol [18]. A recent study in 115 patients showed a clear positive correlation between plasma tramadol concentration and QT duration [19].
Buprenorphine was traditionally considered to be a relatively safe drug in regards to developing long QT syndrome [2021]. However, recent reports indicate that buprenorphine increases the risk of a prolonged QT interval [2223]. Most of the evidence comes from patients undergoing substitution therapy at considerably higher doses than those used in patients being treated for pain. However, the clinical significance of the observed effects is still debated [22].
Oxycodone has a low affinity for the hERG channels. It has been shown to prolong the QT interval in a dose-dependent manner [24]. In a retrospective analysis of 137 patients treated for oxycodone overdoses, 17% had an increased QTc interval [25].
A recent study prospectively analyzed the risk of developing long QT syndrome in patients on newly initiated pethidine treatment and found that up to 25% showed a pathologic prolongation of cardiac repolarization. The QTc and changes from the pre-treatment baseline QTc showed a positive correlation with plasma levels of pethidine and an even stronger correlation with those of the metabolite normeperidine [26].
Lamotrigine blocks hERG potassium channels to a significant extent in vitro, but topiramate and gabapentin have considerably less effect [27]. In healthy volunteers, therapeutic doses of lamotrigine were not associated with significant QT changes [28].
Gabapentin enacarbil, a prodrug of gabapentin, had no effect on cardiac repolarization in healthy volunteers [2930]. In rabbits, therapeutic doses of pregabalin significantly prolonged the QT interval [31]. The clinical relevance of these findings in humans is still unknown because there have been no published clinical reports on the association between most antiepileptic drugs and QT prolongation [3233].
Antidepressants are frequently used in patients with chronic pain for two reasons. Firstly, pain and depression are common co-morbid conditions, and secondly, some antidepressants have analgesic properties, e.g. those used in the treatment of neuropathic pain.
Generally, the increase in QTc is significantly greater for tricyclic antidepressants than for selective serotonin reuptake inhibitors; of the latter agents, citalopram has been demonstrated to cause the greatest increase in QT-interval [34]. There have been many reports of long QT-intervals and TdP in patients receiving citalopram [35]. In 2012, the US Food and Drug Administration addressed this problem in a safety communication [36]. Escitalopram was associated with less QT prolongation. More recent research has not found a correlation between plasma escitalopram levels and QTc duration [37]. In contrast, for amitriptyline, a tricyclic antidepressant that is frequently used to treat patients with pain, this correlation is proven [38].
Among the serotonin-noradrenaline reuptake inhibitors, venlafaxine is associated with the highest risk of long QT syndrome [39]. Concerns about such an association appeared soon after venlafaxine was approved for the treatment of depression [40]. However, no correlation could be found between serum venlafaxine concentration and prolongation of the QTc interval.
There is increasing evidence that the cannabinoids can alleviate chronic pain [43]. In a study performed in healthy volunteers, an oral mucosal spray containing delta-9-tetrahydrocannabinol/cannabidiol had no significant effect on ECG parameters, either in recommended or in supra-therapeutic doses [44]. In vitro experiments for JWH-030, a new synthetic cannabinoid, demonstrated blocking of the hERG channel and therefore potential QT prolongation [45].
Antiemetics are frequently used to treat the nausea and vomiting associated with use of opioid analgesics. It is known that all the first-generation 5-hydroxytryptamine3 (5-HT3) receptor antagonists have the potential to increase the QT interval [48]. In a prospective clinical trial, a single 4 mg intravenous dose of ondansetron prolonged the QTc interval by 19.3 ± 18 ms. In another study, administration of ondansetron resulted in long QT syndrome in 31% of patients with heart failure and 46% of those with acute coronary syndrome, respectively [49]. A systematic review of literature in 2013 identified 60 cases of cardiac arrhythmia after administration of ondansetron [50].
For dolasetron, the US Food and Drug Administration has since released a safety warning addressing the its association with an increased risk of ECG changes, particularly in patients with pre-existing heart disease [51]. For granisetron, evidence remains unequivocal [525354].
Haloperidol is an interesting agent in the treatment of pain, in that it has both antipsychotic and analgesic actions [5556]. Further, it has a well-known antiemetic effect, which is achieved in doses that are considerably lower than shore recommended for psychiatric indications [57]. In a recent study, haloperidol was non-inferior to ondansetron in treatment of postoperative nausea and vomiting; unfortunately, the risk of QT prolongation was comparable [58]. Similarly, other substances in the butyrophenone group, e.g., domperidone and droperidol, have also been shown to have an increased risk of long QT syndrome [5960].
Proton pump inhibitors are among the most frequently prescribed drugs and are overused, especially in long-term therapy [63]. They are known to cause hypomagnesemia because of loss of magnesium via both the renal and gastrointestinal routes [6465]. This mechanism could potentially have contributed in the case-report of a patient who developed life-threatening TdP 7 hours after oral administration of pantoprazole 40 mg [66]. For esomeprazole, no effect on ECG parameters could be found in healthy volunteers 1.5 or 3 hours after a 40 mg dose [67].
In another case report, addition of lansoprazole in a patient already taking disopyramide, a class Ia antiarrhythmic agent, was associated with development of in long QT syndrome (QTc 690 ms) and TdP [68]. There has been an even more dramatic case report of pulseless ventricular tachycardia in a patient who received lansoprazole in addition to long-term therapy with voriconazole, an antifungal agent known to prolong repolarization [69].
Use of diuretic drugs is common, especially in elderly patients. These agents are also the most common cause for drug-induced electrolyte disorders [70]. These adverse effects are dose dependent [71]. Electrolyte disturbances may indirectly increase the risk of TdP [7273]. Further, furosemide has long been known to increase the QT interval [74].
Prolongation of cardiac repolarization is a severe complication caused by many substances. Increasing awareness and ongoing research have raised suspicion regarding several pharmacological agents. However, the clinical significance of single reports and in-vitro experiments is not always clear.
There are three pivotal strategies for reducing the risk of QT prolongation. First, awareness should be raised regarding possible circumstances or preexisting comorbidities that could increase the risk of QT prolongation. Being female, advanced age, hypokalemia, hypomagnesemia, a history of heart failure, and bradycardia are often mentioned in this context. Second, awareness should be increased regarding medications associated with an increased risk of ECG changes. In the field of pain medicine all the major drug classes contain agents that have an increased risk of QT prolongation. Other substances, not used in the treatment of pain, such as proton pump inhibitors, antiemetics, and diuretics are also associated with long QT syndrome (Table 1). Third, when risk factors are identified, alone, or even worse in combination, alternative treatment options should be considered. When the possible benefits of therapy outweigh the associated risks, slow dose titration and ECG monitoring are recommended.
References
1. Kallergis EM, Goudis CA, Simantirakis EN, Kochiadakis GE, Vardas PE. Mechanisms, risk factors, and management of acquired long QT syndrome: a comprehensive review. ScientificWorldJournal. 2012; 2012:212178. PMID: 22593664.

2. Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003; 163:2433–2445. PMID: 14609780.
3. Zemrak WR, Kenna GA. Association of antipsychotic and antidepressant drugs with Q-T interval prolongation. Am J Health Syst Pharm. 2008; 65:1029–1038. PMID: 18499875.

4. Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010; 121:1047–1060. PMID: 20142454.

5. Trinkley KE, Page RL 2nd, Lien H, Yamanouye K, Tisdale JE. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin. 2013; 29:1719–1726. PMID: 24020938.

6. Vandael E, Vandenberk B, Vandenberghe J, Willems R, Foulon V. Risk factors for QTc-prolongation: systematic review of the evidence. Int J Clin Pharm. 2017; 39:16–25. PMID: 28012118.

7. Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, Hill AP. hERG K(+) channels: structure, function, and clinical significance. Physiol Rev. 2012; 92:1393–1478. PMID: 22988594.
8. Koffeman AR, Valkhoff VE, Celik S, W't Jong G, Sturkenboom MC, Bindels PJ, et al. High-risk use of over-the-counter non-steroidal anti-inflammatory drugs: a population-based cross-sectional study. Br J Gen Pract. 2014; 64:e191–e198. PMID: 24686883.

9. Fanelli A, Ghisi D, Aprile PL, Lapi F. Cardiovascular and cerebrovascular risk with nonsteroidal anti-inflammatory drugs and cyclooxygenase 2 inhibitors: latest evidence and clinical implications. Ther Adv Drug Saf. 2017; 8:173–182. PMID: 28607667.

10. Nagele P, Pal S, Brown F, Blood J, Miller JP, Johnston J. Postoperative QT interval prolongation in patients undergoing noncardiac surgery under general anesthesia. Anesthesiology. 2012; 117:321–328. PMID: 22692379.

11. Pathak A, Boveda S, Defaye P, Mansourati J, Mallaret M, Thebault L, et al. Celecoxib-associated torsade de pointes. Ann Pharmacother. 2002; 36:1290–1291. PMID: 12086565.

12. Frolov RV, Ignatova II, Singh S. Inhibition of HERG potassium channels by celecoxib and its mechanism. PLoS One. 2011; 6:e26344. PMID: 22039467.

13. Kristóf A, Husti Z, Koncz I, Kohajda Z, Szél T, Juhász V, et al. Diclofenac prolongs repolarization in ventricular muscle with impaired repolarization reserve. PLoS One. 2012; 7:e53255. PMID: 23300901.

14. Westermeyer J, Adabag S, Anand V, Thuras P, Yoon G, Batres-Y-Carr T. Methadone maintenance dose/weight ratio, long QTc, and EKG screening. Am J Addict. 2016; 25:499–507. PMID: 27548638.

15. Grodofsky S, Edson E, Huang S, Speck RM, Hatchimonji J, Lacy K, et al. The QTc effect of low-dose methadone for chronic pain: a prospective pilot study. Pain Med. 2015; 16:1112–1121. PMID: 25644980.

16. van den Beuken-van Everdingen MH, Geurts JW, Patijn J. Prolonged QT interval by methadone: relevance for daily practice? A prospective study in patients with cancer and noncancer pain. J Opioid Manag. 2013; 9:263–267. PMID: 24353019.

17. Chou R, Cruciani RA, Fiellin DA, Compton P, Farrar JT, Haigney MC, et al. Methadone safety: a clinical practice guideline from the American Pain Society and College on Problems of Drug Dependence, in collaboration with the Heart Rhythm Society. J Pain. 2014; 15:321–337. PMID: 24685458.

18. Emamhadi M, Sanaei-Zadeh H, Nikniya M, Zamani N, Dart RC. Electrocardiographic manifestations of tramadol toxicity with special reference to their ability for prediction of seizures. Am J Emerg Med. 2012; 30:1481–1485. PMID: 22306385.

19. Keller GA, Etchegoyen MC, Fernandez N, Olivera NM, Quiroga PN, Belloso WH, et al. Tramadol induced QTc-interval prolongation: prevalence, clinical factors and correlation to plasma concentrations. Curr Drug Saf. 2016; 11:206–214. PMID: 26916784.

20. Anchersen K, Clausen T, Gossop M, Hansteen V, Waal H. Prevalence and clinical relevance of corrected QT interval prolongation during methadone and buprenorphine treatment: a mortality assessment study. Addiction. 2009; 104:993–999. PMID: 19392907.

21. Stallvik M, Nordstrand B, Kristensen Ø, Bathen J, Skogvoll E, Spigset O. Corrected QT interval during treatment with methadone and buprenorphine--relation to doses and serum concentrations. Drug Alcohol Depend. 2013; 129:88–93. PMID: 23084592.

22. Kao DP, Haigney MC, Mehler PS, Krantz MJ. Arrhythmia associated with buprenorphine and methadone reported to the Food and Drug Administration. Addiction. 2015; 110:1468–1475. PMID: 26075588.

23. Poole SA, Pecoraro A, Subramaniam G, Woody G, Vetter VL. Presence or absence of QTc prolongation in buprenorphine-naloxone among youth with opioid dependence. J Addict Med. 2016; 10:26–33. PMID: 26690291.

24. Fanoe S, Jensen GB, Sjøgren P, Korsgaard MP, Grunnet M. Oxycodone is associated with dose-dependent QTc prolongation in patients and low-affinity inhibiting of hERG activity in vitro. Br J Clin Pharmacol. 2009; 67:172–179. PMID: 19159406.

25. Berling I, Whyte IM, Isbister GK. Oxycodone overdose causes naloxone responsive coma and QT prolongation. QJM. 2013; 106:35–41. PMID: 23023890.

26. Keller GA, Villa Etchegoyen MC, Fernández N, Olivera NM, Quiroga PN, Diez RA, et al. Meperidine-induced QTc-interval prolongation: prevalence, risk factors, and correlation to plasma drug and metabolite concentrations. Int J Clin Pharmacol Ther. 2017; 55:275–285. PMID: 27509828.

27. Danielsson BR, Lansdell K, Patmore L, Tomson T. Effects of the antiepileptic drugs lamotrigine, topiramate and gabapentin on hERG potassium currents. Epilepsy Res. 2005; 63:17–25. PMID: 15716081.

28. Dixon R, Job S, Oliver R, Tompson D, Wright JG, Maltby K, et al. Lamotrigine does not prolong QTc in a thorough QT/QTc study in healthy subjects. Br J Clin Pharmacol. 2008; 66:396–404. PMID: 18662287.

29. Davy M, Upward J, Arumugham T, Twomey C, Chen C, Stier B. Cardiac repolarization with Gabapentin enacarbil in a randomized, double-blind, placebo- and active-controlled, crossover thorough QT/QTc study in healthy adults. Clin Ther. 2013; 35:1964–1974. PMID: 24290737.

30. Chen D, Lal R, Zomorodi K, Atluri H, Ho J, Luo W, et al. Evaluation of gabapentin enacarbil on cardiac repolarization: a randomized, double-blind, placebo- and active-controlled, crossover thorough QT/QTc study in healthy adults. Clin Ther. 2012; 34:351–362.e3. PMID: 22325733.

31. Alp R, Citil M, Uzun M, Alp S, Topcu B, Uzlu E, et al. Effects of therapeutic doses of Pregabalin on QTc interval in conscious rabbits. Eur Rev Med Pharmacol Sci. 2008; 12:223–228. PMID: 18727453.
32. Keller GA, Ponte ML, Di Girolamo G. Other drugs acting on nervous system associated with QT-interval prolongation. Curr Drug Saf. 2010; 5:105–111. PMID: 20210727.

33. Feldman AE, Gidal BE. QTc prolongation by antiepileptic drugs and the risk of torsade de pointes in patients with epilepsy. Epilepsy Behav. 2013; 26:421–426. PMID: 23218812.

34. Beach SR, Kostis WJ, Celano CM, Januzzi JL, Ruskin JN, Noseworthy PA, et al. Meta-analysis of selective serotonin reuptake inhibitor-associated QTc prolongation. J Clin Psychiatry. 2014; 75:e441–e449. PMID: 24922496.

35. Tampi RR, Balderas M, Carter KV, Tampi DJ, Moca M, Knudsen A, et al. Citalopram, QTc prolongation, and torsades de pointes. Psychosomatics. 2015; 56:36–43. PMID: 25619672.

36. FDA Drug Safety Communication. Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses [Internet]. Rockville, MD: Food and Drug Administration;2012. cited 2017 Oct 2. Available at https://www.fda.gov/Drugs/DrugSafety/ucm297391.htm.
37. Carceller-Sindreu M, de Diego-Adeliño J, Portella MJ, Garcia-Moll X, Figueras M, Fernandez-Vidal A, et al. Lack of relationship between plasma levels of escitalopram and QTc-interval length. Eur Arch Psychiatry Clin Neurosci. 2017; 267:815–822. PMID: 28116499.

38. Unterecker S, Pfuhlmann B, Kopf J, Kittel-Schneider S, Reif A, Deckert J. Increase of heart rate and QTc by amitriptyline, but not by venlafaxine, is correlated to serum concentration. J Clin Psychopharmacol. 2015; 35:460–463. PMID: 26035054.

39. Jasiak NM, Bostwick JR. Risk of QT/QTc prolongation among newer non-SSRI antidepressants. Ann Pharmacother. 2014; 48:1620–1628. PMID: 25204465.

40. Letsas K, Korantzopoulos P, Pappas L, Evangelou D, Efremidis M, Kardaras F. QT interval prolongation associated with venlafaxine administration. Int J Cardiol. 2006; 109:116–117. PMID: 16574528.

41. Tarantino P, Appleton N, Lansdell K. Effect of trazodone on hERG channel current and QT-interval. Eur J Pharmacol. 2005; 510:75–85. PMID: 15740727.

42. Burgess CD, Hames TK, George CF. The electrocardiographic and anticholinergic effects of trazodone and imipramine in man. Eur J Clin Pharmacol. 1982; 23:417–421. PMID: 7151845.

43. Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015; 313:2456–2473. PMID: 26103030.
44. Sellers EM, Schoedel K, Bartlett C, Romach M, Russo EB, Stott CG, et al. A multiple-dose, randomized, double-blind, placebo-controlled, parallel-group QT/QTc study to evaluate the electrophysiologic effects of THC/CBD spray. Clin Pharmacol Drug Dev. 2013; 2:285–294. PMID: 27121791.

45. Yun J, Yoon KS, Lee TH, Lee H, Gu SM, Song YJ, et al. Synthetic cannabinoid, JWH-030, induces QT prolongation through hERG channel inhibition. Toxicol Res. 2016; 5:1663–1671.

46. Kaddar N, Vigneault P, Pilote S, Patoine D, Simard C, Drolet B. Tizanidine (Zanaflex): a muscle relaxant that may prolong the QT interval by blocking IKr. J Cardiovasc Pharmacol Ther. 2012; 17:102–109. PMID: 21317414.
47. Yamagiwa T, Morita S, Amino M, Miura N, Saito T, Inokuchi S. Serum concentration of eperisone hydrochloride correlates with QT interval. Am J Emerg Med. 2014; 32:75–77. PMID: 24135462.

48. Kovac AL. Comparative pharmacology and guide to the use of the serotonin 5-HT3 receptor antagonists for postoperative nausea and vomiting. Drugs. 2016; 76:1719–1735. PMID: 27988869.

49. Hafermann MJ, Namdar R, Seibold GE, Page RL 2nd. Effect of intravenous ondansetron on QT interval prolongation in patients with cardiovascular disease and additional risk factors for torsades: a prospective, observational study. Drug Healthc Patient Saf. 2011; 3:53–58. PMID: 22046106.
50. Freedman SB, Uleryk E, Rumantir M, Finkelstein Y. Ondansetron and the risk of cardiac arrhythmias: a systematic review and postmarketing analysis. Ann Emerg Med. 2014; 64:19–25. PMID: 24314899.

51. FDA Drug Safety Communication. Abnormal heart rhythms ass ociated with use of Anzemet (dolasetron mesylate) [Internet]. Rockville, MD: U.S Food and Drug Administration;2010. cited 2017 Oct 2. Available at https://www.fda.gov/Drugs/DrugSafety/ucm237081.htm#safety_announcement.
52. Buyukavci M, Olgun H, Ceviz N. The effects of ondansetron and granisetron on electrocardiography in children receiving chemotherapy for acute leukemia. Am J Clin Oncol. 2005; 28:201–204. PMID: 15803017.

53. Mason JW, Selness DS, Moon TE, O'Mahony B, Donachie P, Howell J. Pharmacokinetics and repolarization effects of intravenous and transdermal granisetron. Clin Cancer Res. 2012; 18:2913–2921. PMID: 22452942.

54. Carmichael J, Harris AL. High-dose i.v. granisetron for the prevention of chemotherapy-induced emesis: cardiac safety and tolerability. Anticancer Drugs. 2003; 14:739–744. PMID: 14551508.

55. Judkins KC, Harmer M. Haloperidol as an adjunct analgesic in the management of postoperative pain. Anaesthesia. 1982; 37:1118–1120. PMID: 7137561.

56. Maltbie AA, Cavenar JO Jr, Sullivan JL, Hammett EB, Zung WW. Analgesia and haloperidol: a hypothesis. J Clin Psychiatry. 1979; 40:323–326. PMID: 222741.
57. Büttner M, Walder B, von Elm E, Tramèr MR. Is low-dose haloperidol a useful antiemetic?: a meta-analysis of published and unpublished randomized trials. Anesthesiology. 2004; 101:1454–1463. PMID: 15564955.
58. Yazbeck-Karam VG, Siddik-Sayyid SM, Barakat HB, Korjian S, Aouad MT. Haloperidol versus ondansetron for treatment of established nausea and vomiting following general anesthesia: a randomized clinical trial. Anesth Analg. 2017; 124:438–444. PMID: 28002167.

59. Rossi M, Giorgi G. Domperidone and long QT syndrome. Curr Drug Saf. 2010; 5:257–262. PMID: 20394569.

60. Calver L, Isbister GK. High dose droperidol and QT prolongation: analysis of continuous 12-lead recordings. Br J Clin Pharmacol. 2014; 77:880–886. PMID: 24168079.

61. Chou CC, Wu D. Torsade de pointes induced by metoclopramide in an elderly woman with preexisting complete left bundle branch block. Chang Gung Med J. 2001; 24:805–809. PMID: 11858397.
62. Siddique SM, Shariff N, Vesuwala N, Hafiz T. Metoclopramide as a possible cause of prolonged QT syndrome and torsade de pointes in a patient with heart failure and renal insufficiency. Ann Intern Med. 2009; 150:502–504. PMID: 19349637.

63. Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk [corrected]. Am J Gastroenterol. 2009; 104(Suppl 2):S27–S32. PMID: 19262544.
64. Gau JT, Yang YX, Chen R, Kao TC. Uses of proton pump inhibitors and hypomagnesemia. Pharmacoepidemiol Drug Saf. 2012; 21:553–559. PMID: 22337212.

65. Danziger J, William JH, Scott DJ, Lee J, Lehman LW, Mark RG, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013; 83:692–699. PMID: 23325090.

66. Bibawy JN, Parikh V, Wahba J, Barsoum EA, Lafferty J, Kowalski M, et al. Pantoprazole (proton pump inhibitor) contributing to torsades de pointes storm. Circ Arrhythm Electrophysiol. 2013; 6:e17–e19. PMID: 23592872.

67. Hasselgren B, Claar-Nilsson C, Hasselgren G, Niazi M, Svernhage E. Studies in healthy volunteers do not show any electrocardiographic effects with esomeprazole. Clin Drug Investig. 2000; 20:425–431.

68. Asajima H, Saito N, Ohmura Y, Ohmura K. Lansoprazole precipitated QT prolongation and torsade de pointes associated with disopyramide. Eur J Clin Pharmacol. 2012; 68:331–333. PMID: 21898101.

69. Tsubokura M, Miura Y, Itokawa T, Murata K, Takei N, Higaki T, et al. Fatal dysrhythmia following initiation of lansoprazole during a long-term course of voriconazole. J Clin Pharmacol. 2011; 51:1488–1490. PMID: 21098688.

70. Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med. 2013; 126:256–263. PMID: 23332973.

71. Papademetriou V. Diuretics, hypokalemia, and cardiac arrhythmia: a 20-year controversy. J Clin Hypertens (Greenwich). 2006; 8:86–92. PMID: 16470076.

72. Singh BN, Hollenberg NK, Poole-Wilson PA, Robertson JI. Diuretic-induced potassium and magnesium deficiency: relation to drug-induced QT prolongation, cardiac arrhythmias and sudden death. J Hypertens. 1992; 10:301–316. PMID: 1316396.

73. Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med. 2004; 27:153–160. PMID: 15261358.

74. Akita M, Kuwahara M, Tsubone H, Sugano S. ECG changes during furosemide-induced hypokalemia in the rat. J Electrocardiol. 1998; 31:45–49. PMID: 9533377.





 PDF
PDF Citation
Citation Print
Print


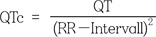

 XML Download
XML Download