Abstract
Background
To identify a new strategy for postoperative pain management, we investigated the analgesic effects of allopregnanolone (Allo) in an incisional pain model, and also assessed its effects on the activities of the primary afferent fibers at the dorsal horn.
Methods
In experiment 1, 45 rats were assigned to Control, Allo small-dose (0.16 mg/kg), and Allo large-dose (1.6 mg/kg) groups (n = 15 in each). The weight bearing and mechanical withdrawal thresholds of the hind limb were measured before and at 2, 24, 48, and 168 h after Brennan's surgery. In experiment 2, 16 rats were assigned to Control and Allo (0.16 mg/kg) groups (n = 8 in each). The degree of spontaneous pain was measured using the grimace scale after the surgery. Activities of the primary afferent fibers in the spinal cord (L6) were evaluated using immunohistochemical staining.
Results
In experiment 1, the withdrawal threshold of the Allo small-dose group was significantly higher than that of the Control group at 2 h after surgery. Intergroup differences in weight bearing were not significant. In experiment 2, intergroup differences in the grimace scale scores were not significant. Substance P release in the Allo (0.16 mg/kg) group was significantly lower than that in the Control group.
Significant improvement has been made in the management of postoperative pain. However, many patients still experience discomfort and side effects resulting in distress, higher morbidity, and prolonged hospital stays [123]. Allopregnanolone (Allo), a neurosteroid, has been shown to exert beneficial effects on pain behaviors or sensitivities in various neuropathic pain models and painful conditions [456789101112]. However, to date, there has been no report addressing the effects of Allo in postoperative pain models.
Kawano et al. [11] reported lower Allo concentrations in the spinal cord of hyperalgesic rats than in those of non-hyperalgesic rats, but not in the brain or serum. Furthermore, intrathecal exogeneous administration of Allo resulted in anti-hyperalgesic effects in hyperalgesic rats following spinal nerve ligation. Therefore, the spinal cord was suggested as an important site for the anti-nociceptive effects of Allo. On the other hand, substance P specifically binds to neurokinin-1 receptors (NK-1r) in the superficial dorsal horn (lamine I and II), causing receptor internalization. This NK-1r internalization is considered as an index of extracellular substance P release from primary afferents and reflects the degree of the primary afferent fiber activities in the spinal dorsal horn. Moreover, NK-1r internalization can be visualized using immunohistochemistry as a quantitative assay for neurotransmitter release [131415].
In the present study, we investigated the effects of Allo in Brennane's postoperative pain model [16], and also assessed its effects on the activity of the primary afferent fibers in the spinal dorsal horn using immunohistochemistry.
This study was approved by the Institutional Animal Care and Use Committee at the University of Tsukuba. A total of 61 male Sprague-Dawleyrats (SLC Ltd, Japan) were used in this study. They were housed under standard conditions (room temperature 24℃, 14/10 h light/dark cycle) with ad labium access to food and water. A 1-week acclimation period was allowed after arrival at the facility.
Allopregnanolone (5α-pregnane-5α-ol-20-one) sulfate was purchased from Steraloids Inc. (Newport, RI). The vehicle solution for Allo contained 5% polysorbate 80 (Wako Pure Chemical Ind. Ltd., Japan) and 16% polyethylene glycol (Sigma, MO) in physiological saline. Allo or vehicle was injected subcutaneously, under the skin of the back, at a volume of 1 ml/kg.
The rat hind paw plantar incision model of postoperative pain was used as previously described [16]. Under 2.5% isoflurane anesthesia, a 1.0 cm longitudinal incision was made with a No. 11 scalpel 0.5 cm from the end of the heel to the base of the toes. After elevation of the flexor tendon, the incision was closed with two mattress sutures of 5-0 nylon on a FS-2 needle. The animals were allowed to recover from general anesthesia in a cage.
Forty-five rats, weighing 222 ± 13 g at the start of the experiment, were assigned randomly to the Control, Allo small-dose (0.16 mg/kg), or Allo large-dose (1.6 mg/kg) group (n = 15 per group). Fifteen minutes before the surgery, each rat received an injection of the vehicle solution or designated dose of Allo. The dose of Allo was selected according to the report of Ocvirk et al. [7].
Hind limb weight bearing and mechanical withdrawal thresholds were measured before surgery (0 h), and at 2, 24, 48, and 168 h after surgery. The unrestrained animals were placed on an elevated plastic mesh floor (grid 12 × 12 mm) under a clear cage (21 × 21 × 15 cm) and allowed to acclimate for 15 min. Each animal was closely observed during a 1-min period. The scores were given depending on the position in which the foot was found during the scoring period: a score of 0 if the foot was completely off the mesh, a score of 1 if the area of the wound touched the mesh without bearing weight, and a score of 2 if the animal put full weight on the foot. The weight bearing score was calculated with the sum of each score × time (sec).
Withdrawal responses to mechanical stimulation were subsequently determined using von Frey filaments (Stoelting, Wood Dale, IL, USA) applied from underneath the cage through an opening in the mesh floor to an area adjacent to the wound. The von Frey set consists of 10 filaments (0.4, 0.6, 1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10.0, and 15.0 g), and the testing is initiated with the 2 g filament. In the absence of a paw withdrawal response (negative response) to the initially selected filament, a stronger stimulus was presented; in the event of an initial paw withdrawal (positive response), the next weaker stimulus was chosen. In cases where continuous positive or negative responses were observed to the exhaustion of the stimulus set, values of 0.4 g and 15 g were assigned respectively. The up-down method was used to record the threshold [17].
Sixteen rats, weighing 260 ± 14 g, were assigned randomly to either the Control or Allo group (n = 8 per group). Fifteen minutes before the plantar incision, each rat received an injection of vehicle solution or Allo (0.16 mg/kg).
At 40 m after the surgery, spontaneous pain degree was measured using the rat grimace scale [18]. The grimace scale consists of the observation of four action units (orbital tightening, nose/cheek flattening, ear changes, and whisker changes) and ranges from 0 (pain free) to 8 (most severe).
At 45 m after the surgery, rats were deeply anesthetized with an intraperitoneal injection of 5% pentobarbital (150 mg/kg) and perfused transcardially with 500 ml of 0.1M phosphate buffered saline (PBS, pH 7.4). Subsequently, the fixation was carried out with 500 ml of 4% formaldehyde in PBS.
The lumbar spinal cord was rapidly dissected and postfixed in the same fixation solution for 2 h After cryoprotective treatment, the spinal segment L6 was cut into 30-µm transverse slices at intervals of 300 µm with a cryostat (Leica, Germany) and analyzed by fluorescence immunohistochemistry as described previously, with minor modifications [19]. Six sections per animal were labeled with primary antiserum, rabbit anti-NK-1r (1:3,000; Advanced Targeting Systems, San Diego, CA) and mouse anti-NeuN (1:1,000, Millipore, Temecula, CA) in PBS containing 10% normal goat serum and 0.2% Triton-X for 18 h at room temperature. After a rinse in PBS, sections were incubated in secondary antibodies, Alexa Fluor 488 Goat anti-rabbit IgG (1:1,000, A11008, Life Technology Japan Ltd., Tokyo, Japan) and Alexa Fluor 594 Goat anti-mouse IgG (1:1,000, A-11032, Life Technology Japan Ltd., Tokyo, Japan) in PBS for 2 h at room temperature. All sections were finally rinsed in PBS, mounted on glass slides, and covered with a coverslip using ProLong Gold Antifade Reagent (Life Technology Japan Ltd., Tokyo, Japan).
Six NeuN-positive cells per section were randomly selected in each side of the dorsal horn (lamina I) and NK-1r internalization was counted at X300 magnification using an Olympus fluorescence microscope (FLUOVIEW FV10i, Olympus Corporation, Tokyo, Japan). Data collection was performed by two authors blinded to the treatment (vehicle or Allo) during the behavioral testing and microscopic examination. Treatment status was subsequently exposed and statistically analyzed.
Pain score and mechanical allodynia were analyzed by using the Kruskal-Wallis test (followed by Steel's test) and Wilcoxon's signed rank test. Body weight was analyzed using analysis of variance (ANOVA) and repeated-measures ANOVA. Grimace score and cell number of positive-internalization were analyzed by using the Mann-Whitney U-test and Fisher exact test, respectively. The level of significance was set at P < 0.05.
At the end of the protocol, the body weights of the animals in the three experimental groups were comparable: the Control group, 306 ± 62 g; the Allo small-dose group, 310 ± 54 g; and the Allo large-dose group, 302 ± 63 g. There was no significant difference in weight increment among the three groups.
The weight bearing scores of all the groups decreased at 2, 24, and 48 h after surgery (P < 0.05 vs 0 h) and returned to the pre-incision level at the end of the study (168 h). There were no significant differences in the weight bearing scores among the three experimental groups at any time point (Table 1).
The withdrawal threshold in the incised foot decreased after the incision in all the groups, but not in the Allo large-dose group at 168 h, (P < 0.05 vs 0 h; Fig. 1). The withdrawal threshold of the Allo small-dose group was significantly higher than that of the Control group at 2 h after the surgery (P < 0.05; Fig. 1).
The median (25–75%) grimace scales of the Control and Allo groups were 5 (2–6.3) and 4 (1.5–5), respectively. There was no significant difference in the grimace scale between the two groups.
Unilateral hind paw plantar incision produced a robust NK-1r internalization in the ipsilateral superficial dorsal horn, but not the contralateral dorsal horn (ipsilateral 104/287, contralateral 15/252; P < 0.05).
Systemic administration of Allo (0.16 mg/kg) alleviated mechanical allodynia at 2 h after incision of the hind paw plantar. This result is in line with previous reports, which investigated the effects of Allo in neuropathic pain models and naive animals [5681011]. The anti-allodynic effects of Allo were not dose-dependent in this study; this finding is also in agreement with those of previous studies [47].
The biphasic anti-allodynic effects of Allo implies that more than one mechanism and/or multiple sites of action may be involved. Allo is known to modulate plural channels and receptors such as gamma-amino butyric acid A (GABAA) and glycine receptors as well as L- and T-type calcium channels [202122]. Allo induced analgesia is mediated by both T-type calcium and GABAA channels [22].
Some literatures report that L-type calcium channel blockers prevent hyperalgesia in chemotherapy-induced neuropathy or opioid-induced hyperalgesia models [2324]. In other words, the activation of L-type calcium channels may decrease Allo induced anti-hyperalgesic effects under some conditions.
On the other hand, Allo stimulates GABA receptors at multiple sites of the nervous system. One potential site of action of GABA agonist is in the dorsal horn of the spinal cord, where GABA suppresses afferent nociceptive input [25]. However, GABA is also involved in the regulation of descending pain modulatory pathways originating from the rostroventral medulla (RVM) and periaqueductal gray (PAG). Increasing GABA-ergic tone at these sites has pronociceptive effects [2627]. Thus, the analgesic effects of Allo might be inhibited by decreasing the tonic descending inhibition from the RVM and PAG at higher doses.
In the large dose (1.6 mg/kg) Allo group, no significant differences in the withdrawal threshold were found between the pre-surgery measurement and 168 h after surgery. This might be due to the neuroprotective effects of Allo [428]. Allo is reported to increase proliferation of rodent and human neural progenitor cells in vitro via GABAA receptor and L-type calcium channel-dependent mechanism [29]. Chronic post-surgical pain remains a major clinical problem and its incidence is reported to range between 10 and 50% [3031]. This result might influence the problem of chronic post-surgical pain.
There was no significant difference in the weight bearing score among the three experimental groups. We speculated that the weight bearing score might be affected by the learning ability of the rats. Thus, we selected the grimace score in experiment 2. However, no significant differences were detected between the Allo and Control groups. The spontaneous pain in the incision model might be lower than that of other pain models with nerve damage. Since NK-1r internalization disappeared in about 30 min [14], we measured the grimace scale at only one point. This might be another reason for the negative result of the grimace scale in this study.
Allo decreased the plantar incision-evoked NK-1r internalization in the dorsal horn (lamina I), which implies the inhibition of substance P release from the primary afferent fiber. The decrease of NK-1r internalization was speculated to be due to Allo-induced facilitation of the GABAA receptor in the spinal dorsal horn and presynaptic inhibition of substance P release from the primary afferent terminal [3233]. The decreased release of substance P is in accord with the anti-allodynia effect of Allo.
In conclusion, systemic administration of Allo alleviated mechanical allodynia in a rat postoperative pain model. Inhibition of NK-1r internalization was observed in the dorsal horn (lamina I) of rats treated with Allo. Our findings propose the therapeutic potential of Allo in the postoperative pain management, however, further investigations will be necessary to address its clinical usefulness.
ACKNOWLEDGEMENTS
The authors thank Professor Shigeki Yamaguchi (Department of Anesthesiology, School of Medicine, Dokkyo Medical University) for arranging this study.
This work was financially supported by the Japanese Society for the Promotion of Science (21591997).
References
1. Tan M, Law LS, Gan TJ. Optimizing pain management to facilitate enhanced recovery after surgery pathways. Can J Anaesth. 2015; 62:203–218. PMID: 25501696.

2. Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003; 97:534–540. PMID: 12873949.

3. Kim SH, Yoon KB, Yoon DM, Kim CM, Shin YS. Patient-controlled epidural analgesia with ropivacaine and fentanyl: experience with 2,276 surgical patients. Korean J Pain. 2013; 26:39–45. PMID: 23342206.

4. Frye CA, Duncan JE. Progesterone metabolites, effective at the GABAA receptor complex, attenuate pain sensitivity in rats. Brain Res. 1994; 643:194–203. PMID: 8032914.

5. Pathirathna S, Todorovic SM, Covey DF, Jevtovic-Todorovic V. 5alpha-reduced neuroactive steroids alleviate thermal and mechanical hyperalgesia in rats with neuropathic pain. Pain. 2005; 117:326–339. PMID: 16150542.

6. Meyer L, Venard C, Schaeffer V, Patte-Mensah C, Mensah-Nyagan AG. The biological activity of 3alpha-hydroxysteroid oxido-reductase in the spinal cord regulates thermal and mechanical pain thresholds after sciatic nerve injury. Neurobiol Dis. 2008; 30:30–41. PMID: 18291663.

7. Ocvirk R, Pearson Murphy BE, Franklin KB, Abbott FV. Antinociceptive profile of ring A-reduced progesterone metabolites in the formalin test. Pain. 2008; 138:402–409. PMID: 18343034.

8. Charlet A, Lasbennes F, Darbon P, Poisbeau P. Fast non-genomic effects of progesterone-derived neurosteroids on nociceptive thresholds and pain symptoms. Pain. 2008; 139:603–609. PMID: 18614289.

9. Patte-Mensah C, Meyer L, Schaeffer V, Mensah-Nyagan AG. Selective regulation of 3 alpha-hydroxysteroid oxidoreductase expression in dorsal root ganglion neurons: a possible mechanism to cope with peripheral nerve injury-induced chronic pain. Pain. 2010; 150:522–534. PMID: 20605070.

10. Meyer L, Patte-Mensah C, Taleb O, Mensah-Nyagan AG. Allopregnanolone prevents and suppresses oxaliplatin-evoked painful neuropathy: multi-parametric assessment and direct evidence. Pain. 2011; 152:170–181. PMID: 21071147.

11. Kawano T, Soga T, Chi H, Eguchi S, Yamazaki F, Kumagai N, et al. Role of the neurosteroid allopregnanolone in the hyperalgesic behavior induced by painful nerve injury in rats. J Anesth. 2011; 25:942–945. PMID: 21879341.

12. Afrazi S, Esmaeili-Mahani S. Allopregnanolone suppresses diabetes-induced neuropathic pain and motor deficit through inhibition of GABAA receptor down-regulation in the spinal cord of diabetic rats. Iran J Basic Med Sci. 2014; 17:312–317. PMID: 24967058.
13. Marvizón JC, Wang X, Matsuka Y, Neubert JK, Spigelman I. Relationship between capsaicin-evoked substance P release and neurokinin 1 receptor internalization in the rat spinal cord. Neuroscience. 2003; 118:535–545. PMID: 12699788.

14. Mantyh PW. Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry. 2002; 63(Suppl 11):6–10. PMID: 12562137.
15. Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, et al. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci. 2005; 25:3651–3660. PMID: 15814796.

16. Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996; 64:493–501. PMID: 8783314.

17. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63. PMID: 7990513.

18. Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011; 7:55. PMID: 21801409.

19. Takasusuki T, Yaksh TL. Regulation of spinal substance P release by intrathecal calcium channel blockade. Anesthesiology. 2011; 115:153–164. PMID: 21577088.

20. Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005; 6:565–575. PMID: 15959466.

21. Maksay G, Laube B, Betz H. Subunit-specific modulation of glycine receptors by neurosteroids. Neuropharmacology. 2001; 41:369–376. PMID: 11522328.

22. Pathirathna S, Brimelow BC, Jagodic MM, Krishnan K, Jiang X, Zorumski CF, et al. New evidence that both T-type calcium channels and GABAA channels are responsible for the potent peripheral analgesic effects of 5alpha-reduced neuroactive steroids. Pain. 2005; 114:429–443. PMID: 15777868.

23. Kawashiri T, Egashira N, Kurobe K, Tsutsumi K, Yamashita Y, Ushio S, et al. L type Ca2± channel blockers prevent oxaliplatin-induced cold hyperalgesia and TRPM8 overexpression in rats. Mol Pain. 2012; 8:7. PMID: 22292988.
24. Dogrul A, Bilsky EJ, Ossipov MH, Lai J, Porreca F. Spinal L-type calcium channel blockade abolishes opioid-induced sensory hypersensitivity and antinociceptive tolerance. Anesth Analg. 2005; 101:1730–1735. PMID: 16301251.

25. Jasmin L, Wu MV, Ohara PT. GABA puts a stop to pain. Curr Drug Targets CNS Neurol Disord. 2004; 3:487–505. PMID: 15578966.

26. Roychowdhury SM, Fields HL. Endogenous opioids acting at a medullary mu-opioid receptor contribute to the behavioral antinociception produced by GABA antagonism in the midbrain periaqueductal gray. Neuroscience. 1996; 74:863–872. PMID: 8884782.

27. Gilbert AK, Franklin KB. GABAergic modulation of descending inhibitory systems from the rostral ventromedial medulla (RVM). Dose-response analysis of nociception and neurological deficits. Pain. 2001; 90:25–36. PMID: 11166967.

28. Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, et al. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2010; 107:6498–6503. PMID: 20231471.

29. Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005; 25:4706–4718. PMID: 15888646.

30. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006; 367:1618–1625. PMID: 16698416.

31. Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008; 101:77–86. PMID: 18434337.

32. Zhang Y, Zhao S, Rodriguez E, Takatoh J, Han BX, Zhou X, et al. Identifying local and descending inputs for primary sensory neurons. J Clin Invest. 2015; 125:3782–3794. PMID: 26426077.

33. Witschi R, Punnakkal P, Paul J, Walczak JS, Cervero F, Fritschy JM, et al. Presynaptic alpha2-GABAA receptors in primary afferent depolarization and spinal pain control. J Neurosci. 2011; 31:8134–8142. PMID: 21632935.

Fig. 1
Summary of withdrawal thresholds to von Frey filaments before and after surgery. The results are expressed as medians with 1st and 3rd quartiles, and 10th and 90th percentiles. White column: Control group, Light gray column: Allopregnanolone 0.16 mg/kg group, Dark gray column: Allopregnanolone 1.6 mg/kg group. *P < 0.05 (Control vs Allopregnanolone 0.16 mg/kg group) by Kruskal-Wallis test and Steel's test. #P < 0.05 (vs pre-incision values) by Wilcoxon's signed rank test.
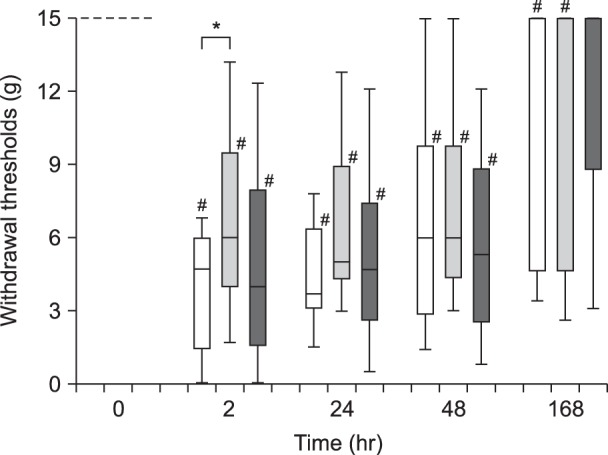
Fig. 2
Positive cell number of neurokinin-1 receptor (Nk-1r) internalization (gray column) and total observation cell number (gray + white column) at 45 minutes after surgery. Cont: Control group, Allo: allopregnanolone (0.16 mg/kg) group. *P < 0.05 vs Control group, #P < 0.05 vs Contralateral side by Fisher exact test.
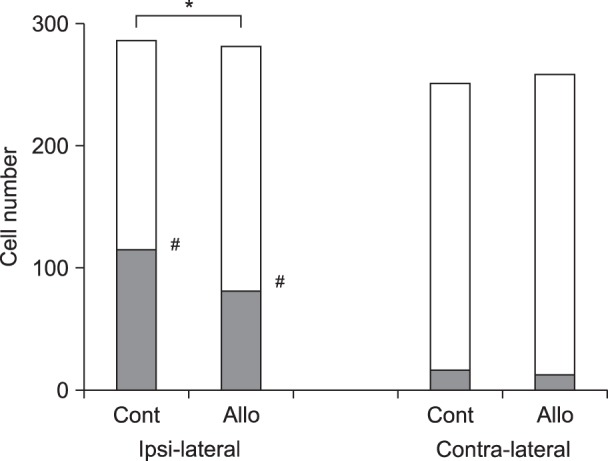
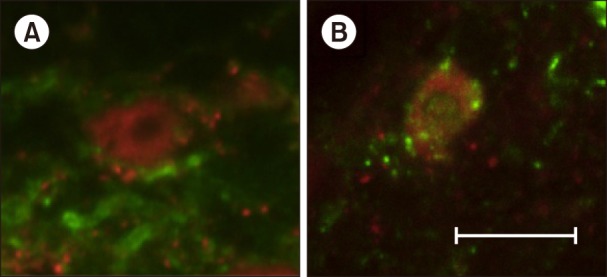
Fig. 3
Representative confocal fluorescent microscopic images of neurokinin-1 receptor (NK-1r) and NeuN at L6 lumbar spinal dorsal horn from incision model rats. Green: NK-1r, Red: NeuN. (A) An image of NK-1r in the lamina I. Notice the presence of NK-1r on a neuron membrane and the lack of NK-1r containing endosomes in the cytoplasm. (B) An image of incision-induced NK-1r internalization. Notice the presence of NK-1r containing endosomes in the cytoplasm. Scale bar is 10 µm.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download