Abstract
Background
To achieve a prolonged therapeutic effect in patients with lumbar facet joint syndrome, radiofrequency medial branch neurotomy (RF-MB) is commonly performed. The purpose of this study was to evaluate the prognostic value of paravertebral muscle twitching when performing RF-MB in patients with lumbar facet joint syndrome.
Methods
We collected and analyzed data from 68 patients with confirmed facet joint syndrome. Sensory stimulation was performed at 50 Hz with a 0.5 V cut-off value. Patients were divided into 3 groups according to the twitching of the paravertebral muscle during 2 Hz motor stimulation: ‘Complete’, when twitching was observed at all needles; ‘Partial’, when twitching was present at 1 or 2 needles; and ‘None’, when no twitching was observed. The relationship between the long-term effects of RF-MB and paravertebral muscle twitching was analyzed.
Results
The mean effect duration of RF-MB was 4.6, 5.8, and 7.0 months in the None, Partial, and Complete groups, respectively (P = 0.47). Although the mean effect duration of RF-MB did not increase significantly in proportion to the paravertebral muscle twitching, the Complete group had prolonged effect duration (> 6 months) than the None group in subgroup analysis. (P = 0.03).
Facet joint syndrome is a common cause of lumbar back pain [123456]. To diagnose and treat patients with facet joint syndrome, intra-articular facet joint injections or medial branch blocks (MBB) are performed [45]. These procedures are also performed to predict the efficacy of denervation treatment prior to facet joint denervation [36]. Radiofrequency medial branch neurotomy (RF-MB) is commonly performed in patients with facet joint syndrome to achieve the prolonged effects of facet joint denervation.
During the RF-MB procedure, the most important objective means of confirming the safe and precise needle position is the radiologic finding [789]. Nerve stimulation is also a useful method for detecting whether the electrode is close to the nerve. However, this method depends on the patient's subjective sensation of a 50-Hz stimulus rather than on an objective measurement. In addition, when the adjacent peripheral nerves that converge into the same nerve root are stimulated, similar sensory provocation may be produced as referred pain [7].
The medial branch nerves in the lumbar spine are sensory nerves that innervate the facet joints of the lumbar spine. In addition to functioning as sensory nerves, these nerves also act as motor nerves that innervate the paravertebral muscles, including the multifidus, iliocostalis, and longissimus muscles [8]. When the electrode stimulates the medial branch to exclude the involvement of the sensory-motor nerve innervating the lower extremity, sensory provocation and twitching of the paravertebral muscle may be observed [39].
However, to the best of our knowledge, there are no previous studies that investigate the relationship between paravertebral muscle twitching and the prognosis of patients undergoing RF-MB. Therefore, the aim of this study was to evaluate the prognostic value of paravertebral muscle twitching in patients with lumbar facet syndrome who had undergone RF-MB.
This retrospective case-control study was approved by the Institutional Review Board (Approval No.: 3-2015-0189) of Gangnam Severance Hospital, Seoul, Korea, and registered at clinicaltrials.gov (NCT02580383). We collected data from 120 patients who were diagnosed with facet joint syndrome and had undergone lumbar RF-MB.
Patient consent to review their medical records was not required by the Institutional Review Board of Gangnam Severance Hospital, because patient identification data were encoded and scrambled using a restricted computer to protect the privacy of all subjects.
Patients without 12-month follow-up data, those who underwent bilateral RF-MB, and those who underwent spine surgery or other interventional procedures during the follow-up period were excluded due to the potential effects of these procedures on pain derived from the lumbar facet joint.
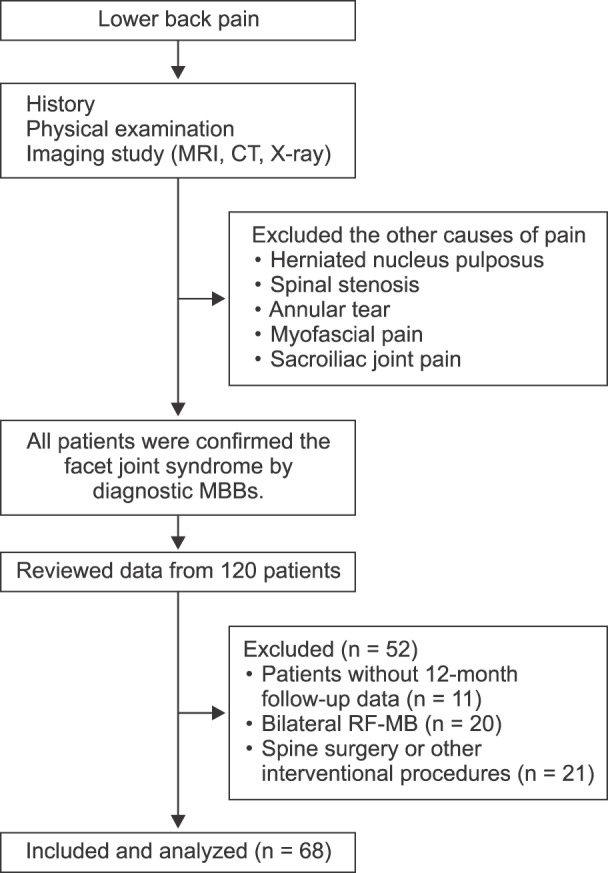
Fig. 1 shows the flow diagram of patient allocation. Patients who had other painful conditions before enrollment were treated with interventional procedures in the lower back. The diagnosis and determination of target nerves were made first with physical examinations such as paravertebral tenderness or pain at facet loading. The diagnosis was confirmed with radiographic evidence of facet degeneration on Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) [1011]. Diagnostic MBBs were performed at two medial branches supplying the target level. Once the needle position was confirmed, 0.3 ml of 0.5% bupivacaine was injected at each site after negative aspiration of the blood. The diagnosis was confirmed after 2 or more sequential diagnostic MBBs. When indicated, patients underwent RF-MB.
RF-MB was performed in patients whose numeric rating scale (NRS; 0 - no pain, 10 - worst-imaginable pain) score decreased to less than half of the initial NRS score after the diagnostic MBB. The procedure was not performed in patients who experienced prolonged or short effects (> 3 days or < 5 h) after the diagnostic MBB in consideration of the effective duration of local anesthetics [12].
RF-MB was performed with the patient in a prone position. Before insertion of the RF needle, a 22-gauge needle was inserted as a guide along the pathway of the target medial branch, similar to the procedure for the diagnostic MBB. After needle placement, the fluoroscopy views were positioned in the ipsilateral-oblique and caudal-cephalad directions to ensure adequate RF needle placement. Consequently, a 10 cm long, 10-mm active-tip curved RF needle with a 20-gauge external diameter was advanced under fluoroscopic guidance to place the needle close to and in parallel with the target medial branch. With the assistance of the prepositioned guide needle, the RF needle was placed in the groove between the transverse and superior articular processes, maximizing the contact area between the active tip and the groove (Fig. 2A and 2B). For the L5 dorsal rami, the needle was advanced through a groove between the sacral ala and the articular process (Fig. 2C and 2D). When positioning the needle, the needle was advanced carefully so that it did not pass the anterior border of the superior articular process in the lateral fluoroscopic view.
After the needle was in position, the sensory stimulation at a frequency of 50 Hz was provided. The stimulation was started at 0.1 V and slowly increased up to 0.5 V. If provocation was not observed until the cutoff value of 0.5 V. The needle was repositioned. In sequence, the stimulation at a frequency of 2 Hz was performed to within double the voltage level at which sensory provocation or the cutoff value of 1 V was acquired. When the contraction of the paravertebral muscle was observed, the voltage level was recorded. If sensory or motor provocation was noted in the lower extremities, the needle was repositioned. After confirming that the needle was in position, 1 ml of 2% mepivacaine mixed with 1 mg of dexamethasone was injected through the guide needle. RF lesioning was performed twice using an RF generator (Pain Management Generator 230V PMG-230; Baylis Medical, Montreal, Canada), which was able to maintain an 80℃ lesioning temperature for 75 s.
One hundred twenty patients were confirmed with the facet joint syndrome and enrolled in this study. All the diagnostic and therapeutic procedures were performed by the same physician in this study. After the RF-MB, the duration of the effective block was also recorded. Patients were followed up for 12 months after the RF-MB. If the patient's maximum numeric pain intensity score decreased to less than half of the initial pain score, then the procedure was regarded as effective. The patients' pain intensity using the NRS (scores ranging from 0 to 10) and the duration of the effective period were investigated and recorded for each patient at 3, 6, and 12 months after the procedure. During the follow up period, only conservative oral medication (nonsteroidal anti-inflammatory drugs) was prescribed to patients.
The patients were grouped according to the adequacy of the RF needle position when performing RF-MB as follows: “Complete,” when paravertebral muscle twitching was observed at all needles; “Partial,” when twitching was observed at 1 or 2 of the needles; and “None,” when no twitching was observed for any of the needles.
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 18.0 software (SPSS Inc., Chicago, IL, USA). ANOVA was used for analysis of the long-term effect of RF-MB.
For a subgroup analysis, regrouping was performed according to the different voltage level ratios. The ratio was set by adjusting the criteria of adequate needle positioning as paravertebral muscle twitching was observed within different multipliers of the voltage level where sensory provocation was observed (1.0 to 2.0 in 0.1 intervals).
To determine the best cut off ratio for the paravertebral twitching/sensory stimulation voltage level, univariate logistic regression was performed for each cutoff value. The best cutoff ratio was determined as a value that has the lowest P value for discriminating muscle twitching on long term effect of RF-MB.
After determination of the best cutoff ratio, multivariate analysis using logistic regression was performed to identify the predictors of the long-term effects of RF-MB. P values of < 0.05 were considered statistically significant. Additionally, 95% confidence intervals for each odds ratio were calculated.
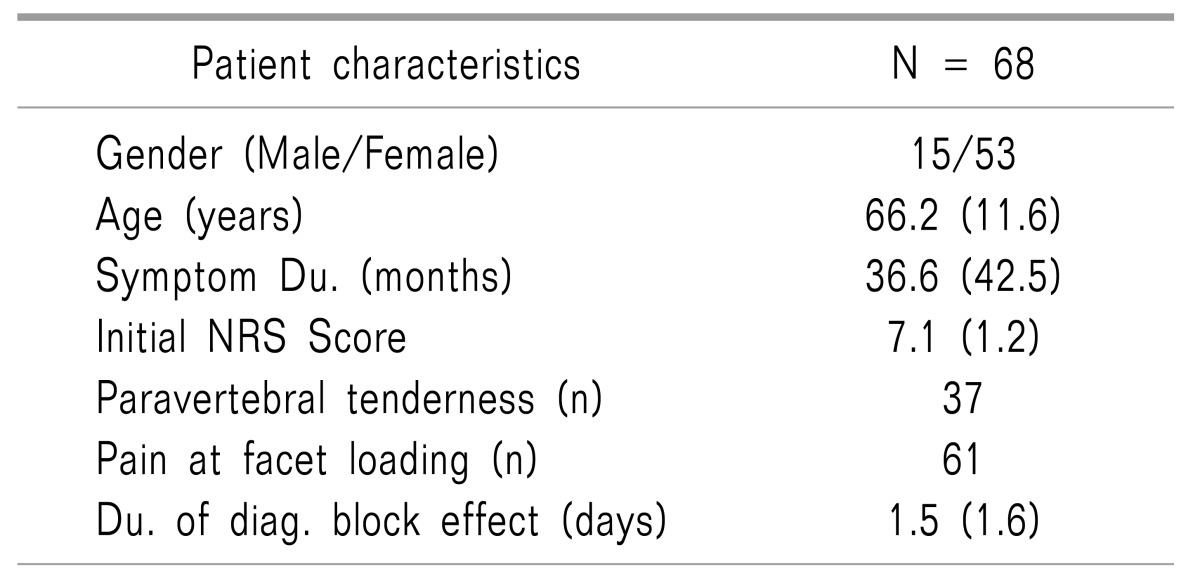
From the 120 patients who underwent RF-MB, 52 patients were excluded due to absent 12-month follow-up data (n = 11), bilateral RF-MB procedure (n = 20), and spine surgery or other interventional procedures after the procedure (n = 12). A total of sixty-eight patients were enrolled in the present study; the demographic data are listed in Table 1. The mean effect duration of RF-MB was 6.0 ± 4.6 months.
The mean effect duration was 4.6, 5.8, and 7.0 months in the None, Partial, and Complete groups, respectively. However, the values were not statistically significant (Fig. 3). In contrast, in an analysis using different cutoff values, the paravertebral muscle twitching/sensory provocation ratio of 1.6 had the lowest P value for discriminating the differences in the long-term effects of RF-MB between the groups (Table 2).
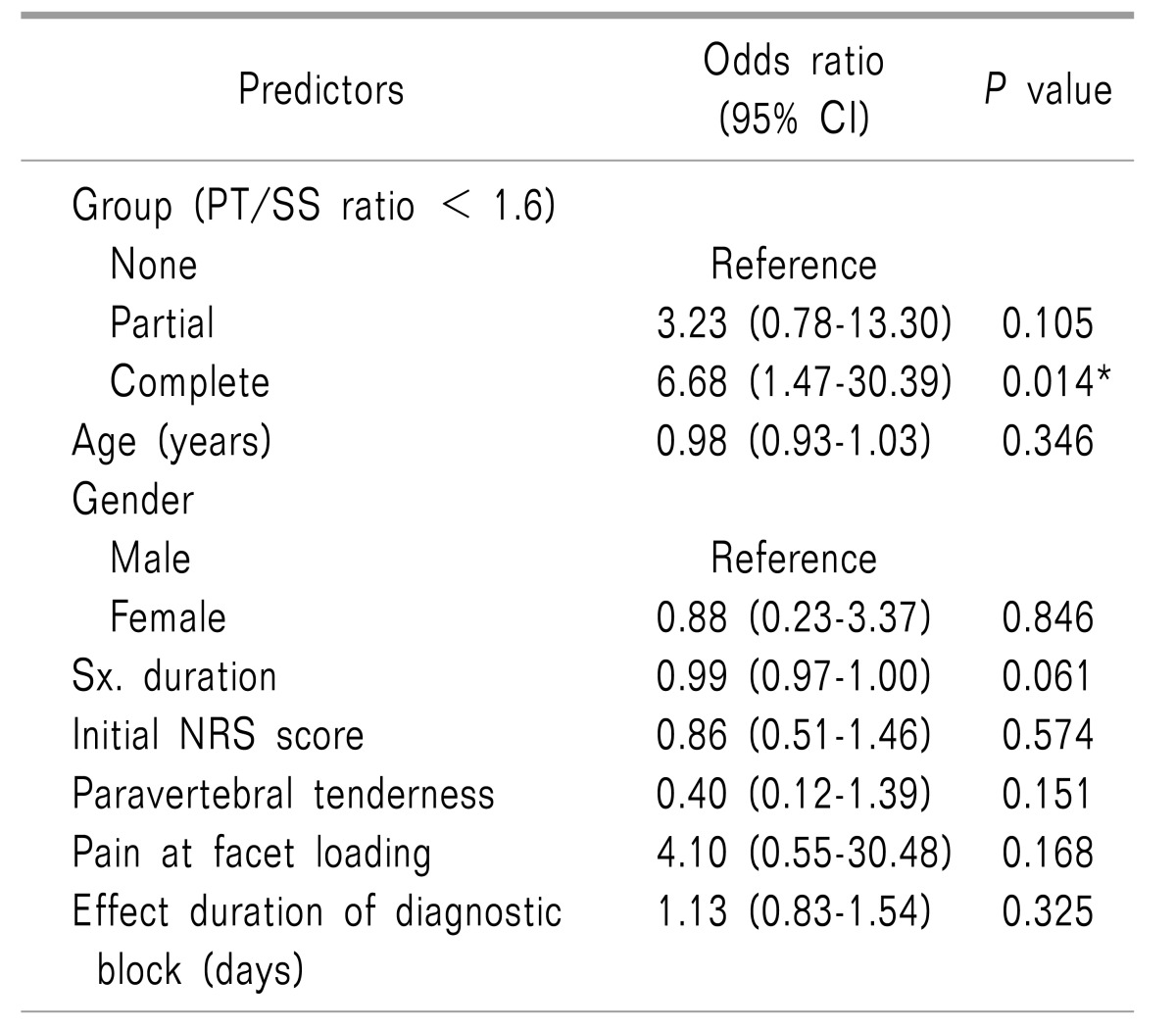
When using this cutoff value of 1.6, there were 21, 23, and 24 patients in the None, Partial and Complete groups, respectively. Multivariate logistic regression analysis, which included various factors that can affect the outcome of RF-MB, was performed. Compared to the None group, the Complete group had a significant odds ratio of 6.68 predicting the long-term efficacy of RF-MB.
Age, gender, pre-procedural pain duration, initial NRS, paravertebral tenderness, pain at facet loading, and the duration of the effect of the diagnostic block were not statistically related to the long-term effects of RF-MB (Table 3).
According to the results of the present study, the mean effect duration of RF-MB did not increase significantly when more paravertebral muscle twitching was observed during the procedure. However, when using different cutoff values, higher probability of a longer effect duration (> 6 months) was expected when paravertebral muscle twitching was observed at every RF-MB needle with a statistically significant difference. When we performed the sensory and motor stimulation at 50 Hz with a 0.5 V cutoff value and at 2 Hz with a 1 V cutoff value, respectively, the findings of the paravertebral muscle twitch may have possibility as a predictive factor in RF-MB.
When performing RF denervation, a spheroid-shaped lesion is created, with its long axis parallel to the RF needle. Thus, when the needle position is not close enough or parallel to the target medial branch, sufficient nerve denervation cannot be achieved [1314]. In addition to using correct anatomical landmark based techniques, sensory stimulation has often been used to determine the proximity of the RF needle to the target nerve [34]. However, using sensory stimulation as the only method of identifying the RF needle proximity is unreliable for several reasons. First, the stimulation of the ligaments, muscles, or periosteum near the target nerve can produce similar sensory provocation [7]. Sensory conduction block, which occurs when the nerve is located close to the electrode needle, may also result in misinterpretations [15].
In addition, demographic, cultural, or psychological factors decrease the reliability of sensory stimulation [16]. Therefore, the presence of paravertebral muscle twitching during RF-MB has been used as an adjuvant method, and positive outcomes have been reported [17].
The method of identifying paravertebral muscle twitching during RF-MB has several advantages. For instance, this more objective measure of needle proximity is more reliable for both patients and clinicians. In addition, this method could be utilized with inpatients who are sedated, intellectually handicapped, or unable to communicate. One report noted that long-lasting effects could be achieved after confirming denervation at the multifidus muscle following RF-MB by monitoring the action potentials [18].
However, the method of identifying paravertebral muscle twitching also has its disadvantages when used as the primary means of needle positioning during RF-MB. Visually identifying paravertebral muscle twitching through cutaneous skin movement is problematic, as other muscles or nerves that are located near the target nerve produce similar twitching when stimulated [819]. [For example, the iliocostalis lumborum or longissimus muscle twitching that is evoked by the lateral or intermediate branch nerve stimulation mimics the multifidus muscle twitching evoked by the medial branch nerve stimulation. In addition, observing the paravertebral muscle twitching may be difficult in patients with paravertebral muscle atrophy or obesity [202122]. Hence, we used the method of identifying the paravertebral muscle twitching as an adjuvant only when sensory provocation at < 0.5 V was confirmed [23].
The best cutoff value for sensory provocation during RF-MB has not been firmly established. However, when sensory provocation was performed at values of < 0.5 V, no statistical differences were noted between the voltage level and therapeutic outcomes. Therefore, a cutoff value of 0.5 V was used when performing the sensory provocation in the present study.
There have been several studies investigating the prognostic factors of positive outcomes after RF-MB. The results suggested that strict indication criteria and adequate diagnostic blocks appear to be closely related to the outcome of RF-MB. While the patient's symptoms and physical examinations may provide some evidence, studies suggest that diagnostic blocks are indispensable for making a reliable diagnosis of facet joint syndrome. Direct facet joint intra-articular injection and MBB are the two most commonly used diagnostic blocks for facet joint syndrome, although research suggests that MBB is more reliable than facet joint intra-articular injection [24]. However, the methodologies used by clinicians when performing or interpreting the results of diagnostic MBBs often differ. For example, Hooten et al. [22] recommended using a comparative MBB with different local anesthetics. The volume of local anesthetics used for diagnostic MBBs also vary, ranging from 0.3 to 1.0 ml. However, as previous studies have reported incidences of motor block in outpatient settings [925], we used less than 0.5 ml of 0.5% bupivacaine in this study. Additionally, clinicians use different cutoff values for determining the level of pain relief after the diagnostic block.
Bogduk recommended that pain relief of more than 80% should be used as a cutoff value after the diagnostic block [25], while Derby et al. [26] recommended a cutoff value of 80% for single MBBs and 70% for double MBBs. In contrast, Cohen et al. [3] reported no significant benefits when the cutoff values for pain relief after diagnostic MBBs were higher than 50%.
Furthermore, it was difficult for some patients to express whether they experienced a decrease in their pain intensity according to exact values such as 70% or 80%. In the present study, we used a cutoff value of 50% for pain relief experienced by the patient after the diagnostic MBB. Thus, by using a cutoff value that could be assessed using the word “half,” we attempted to increase patient compliance and understanding.
In the present study, we also investigated the prognostic value of various demographic factors. Though not statistically significant, we observed a negative correlation between the female sex and RF-MB outcome. A higher proportion of women may experience pain that is > 50% of their initial pain after RF-MB due to their greater sensitivity to pain relative to men [18]. This might be one of the reasons that the overall outcome of this study was not as effective as in the previous literature [927]. We also examined the pain duration before the procedure and the patients' baseline NRS scores. No statistically significant relationships were noted between either the pain duration or baseline NRS and RF-MB outcome. Furthermore, the presence of paravertebral tenderness, pain at facet loading, and effective diagnostic block duration were not related to the RF-MB outcome in the present study. However, although no statistically significant correlations were observed, the positive or negative directions of the odds ratios for these factors in our study were similar to the values reported in other studies [28]. Therefore, studies that include more patients and employ controlled designs may be able to identify statistically significant results for these factors.
As mentioned previously, only a few statistically significant prognostic factors can be monitored or adjusted while performing RF-MB. However, the results of the present study suggest that a better outcome could be anticipated when paravertebral muscle twitching is encountered during RF-MB in combination with sensory provocation at < 0.5 V. Therefore, by attempting to achieve both sensory stimulation and paravertebral muscle twitching during the procedure, we may be able to increase the therapeutic effects of RF-MB.
In addition, patients with paravertebral muscle twitching may have preserved muscular structures supporting the spine, which make conservative rehabilitation therapies more feasible [2125]. Therefore, complete paravertebral muscle twitching may indicate not only the correct RF needle position but also the amount of paravertebral muscle strength needed to preserve the vertebral column and facet joint.
The present study has several limitations. This is a retrospective study and the pre/post-procedural medical or psychological factors were not controlled. Actually, almost all patients in this study had chronic degenerative musculoskeletal pain and they had been taking several analgesics, which was not changed after the procedure. However, during the follow up period, the participants were asked to notify their aggravated or improved facet joint pain. Moreover, while we regularly monitored the pain profile of each patient after the procedure, bias may have been introduced as these pain ratings were dependent upon the patient's memory regarding their NRS scores.
The best cutoff value of voltage levels used in the subgroup analysis also might be calculated differently if RF-MB was performed using different methods. However, by using the results of this study as reference data, we expect that further studies might provide more reliable information for the prediction of prognosis of RF-MB in clinical settings.
In conclusion, paravertebral muscle twitching while performing lumbar RF-MB may be a reliable predictor of long-term efficacy when sensory provocation under 0.5 V is achieved. However, further investigation may be necessary for clarifying its clinical significance.
References
1. Beresford ZM, Kendall RW, Willick SE. Lumbar facet syndromes. Curr Sports Med Rep. 2010; 9:50–56. PMID: 20071922.

2. Cavanaugh JM, Ozaktay AC, Yamashita T, Avramov A, Getchell TV, King AI. Mechanisms of low back pain: a neurophysiologic and neuroanatomic study. Clin Orthop Relat Res. 1997; 166–180.

3. Cohen SP, Strassels SA, Kurihara C, Griffith SR, Goff B, Guthmiller K, et al. Establishing an optimal “cutoff” threshold for diagnostic lumbar facet blocks: a prospective correlational study. Clin J Pain. 2013; 29:382–391. PMID: 23023310.

4. Lakemeier S, Lind M, Schultz W, Fuchs-Winkelmann S, Timmesfeld N, Foelsch C, et al. A comparison of intraarticular lumbar facet joint steroid injections and lumbar facet joint radiofrequency denervation in the treatment of low back pain: a randomized, controlled, double-blind trial. Anesth Analg. 2013; 117:228–235. PMID: 23632051.

5. Manchikanti L, Manchikanti KN, Manchukonda R, Cash KA, Damron KS, Pampati V, et al. Evaluation of lumbar facet joint nerve blocks in the management of chronic low back pain: preliminary report of a randomized, double-blind controlled trial: clinical trial NCT00355914. Pain Physician. 2007; 10:425–440. PMID: 17525777.
6. Rocha ID, Cristante AF, Marcon RM, Oliveira RP, Letaif OB, Barros Filho TE. Controlled medial branch anesthetic block in the diagnosis of chronic lumbar facet joint pain: the value of a three-month follow-up. Clinics (Sao Paulo). 2014; 69:529–534. PMID: 25141111.

7. Shin KM, Choi SE, Yun SH, Lim SY, Jung BH, Lee KH, et al. New more reliable indicator for confirmation of the medial branch in radiofrequency neurotomy. J Korean Pain Soc. 2000; 13:242–246.
8. Bogduk N, Wilson AS, Tynan W. The human lumbar dorsal rami. J Anat. 1982; 134:383–397. PMID: 7076562.
9. Dreyfuss P, Halbrook B, Pauza K, Joshi A, McLarty J, Bogduk N. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophysial joint pain. Spine (Phila Pa 1976). 2000; 25:1270–1277. PMID: 10806505.

10. Zhou X, Liu Y, Zhou S, Fu XX, Yu XL, Fu CL, et al. The correlation between radiographic and pathologic grading of lumbar facet joint degeneration. BMC Med Imaging. 2016; 16:27. PMID: 27025987.

11. Wilde VE, Ford JJ, McMeeken JM. Indicators of lumbar zygapophyseal joint pain: survey of an expert panel with the Delphi technique. Phys Ther. 2007; 87:1348–1361. PMID: 17684091.

12. Fenten MG, Schoenmakers KP, Heesterbeek PJ, Scheffer GJ, Stienstra R. Effect of local anesthetic concentration, dose and volume on the duration of single-injection ultrasound-guided axillary brachial plexus block with mepivacaine: a randomized controlled trial. BMC Anesthesiol. 2015; 15:130. PMID: 26423050.

13. Choi EJ, Choi YM, Jang EJ, Kim JY, Kim TK, Kim KH. Neural ablation and regeneration in pain practice. Korean J Pain. 2016; 29:3–11. PMID: 26839664.

14. Kweon TD, Kim JY, Lee HY, Kim MH, Lee YW. Anatomical analysis of medial branches of dorsal rami of cervical nerves for radiofrequency thermocoagulation. Reg Anesth Pain Med. 2014; 39:465–471. PMID: 25304480.

15. Li J, Kong X, Gozani SN, Shi R, Borgens RB. Current-distance relationships for peripheral nerve stimulation localization. Anesth Analg. 2011; 112:236–241. PMID: 20966439.

16. Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL 3rd. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009; 10:447–485. PMID: 19411059.

17. van Kleef M, Barendse GA, Kessels A, Voets HM, Weber WE, de Lange S. Randomized trial of radiofrequency lumbar facet denervation for chronic low back pain. Spine (Phila Pa 1976). 1999; 24:1937–1942. PMID: 10515020.

18. Kanchiku T, Imajo Y, Suzuki H, Yoshida Y, Nishida N, Taguchi T. Percutaneous radiofrequency facet joint denervation with monitoring of compound muscle action potential of the multifidus muscle group for treating chronic low back pain: a preliminary report. J Spinal Disord Tech. 2014; 27:E262–E267. PMID: 25137144.
19. Baek SO, Ahn SH, Jones R, Cho HK, Jung GS, Cho YW, et al. Activations of deep lumbar stabilizing muscles by transcutaneous neuromuscular electrical stimulation of lumbar paraspinal regions. Ann Rehabil Med. 2014; 38:506–513. PMID: 25229029.

20. Cohen SP, Strassels SA, Kurihara C, Lesnick IK, Hanling SR, Griffith SR, et al. Does sensory stimulation threshold affect lumbar facet radiofrequency denervation outcomes? A prospective clinical correlational study. Anesth Analg. 2011; 113:1233–1241. PMID: 21918166.

21. Wallwork TL, Stanton WR, Freke M, Hides JA. The effect of chronic low back pain on size and contraction of the lumbar multifidus muscle. Man Ther. 2009; 14:496–500. PMID: 19027343.

22. Hooten WM, Martin DP, Huntoon MA. Radiofrequency neurotomy for low back pain: evidence-based procedural guidelines. Pain Med. 2005; 6:129–138. PMID: 15773877.

23. Veizi E, Hayek S. Interventional therapies for chronic low back pain. Neuromodulation. 2014; 17(Suppl 2):31–45. PMID: 25395115.

24. Cohen SP, Moon JY, Brummett CM, White RL, Larkin TM. Medial branch blocks or intra-articular injections as a prognostic tool before lumbar facet radiofrequency denervation: a multicenter, case-control study. Reg Anesth Pain Med. 2015; 40:376–383. PMID: 26066382.

25. Bogduk N. Evidence-informed management of chronic low back pain with facet injections and radiofrequency neurotomy. Spine J. 2008; 8:56–64. PMID: 18164454.

26. Derby R, Melnik I, Lee JE, Lee SH. Correlation of lumbar medial branch neurotomy results with diagnostic medial branch block cutoff values to optimize therapeutic outcome. Pain Med. 2012; 13:1533–1546. PMID: 23126379.

27. MacVicar J, Borowczyk JM, MacVicar AM, Loughnan BM, Bogduk N. Lumbar medial branch radiofrequency neurotomy in New Zealand. Pain Med. 2013; 14:639–645. PMID: 23279154.

28. Holz SC, Sehgal N. What is the correlation between facet joint radiofrequency outcome and response to comparative medial branch blocks? Pain Physician. 2016; 19:163–172. PMID: 27008290.
Fig. 2
Fluoroscopic images of the radio-frequency (RF) needle position. A 20-gauge radiofrequency needle (R) was placed following the insertion of a guide needle (G) so that itis passed the point of guide needle and lies parallel/close to the target medial branch. (A, B) The anteroposterior/lateral view of the L4/5 level facet joint neurotomy. (C, D) The anteroposterior/lateral view of the L5/S1 level facet joint neurotomy. AP: anteroposterior, OBL: oblique, LAT: lateral.
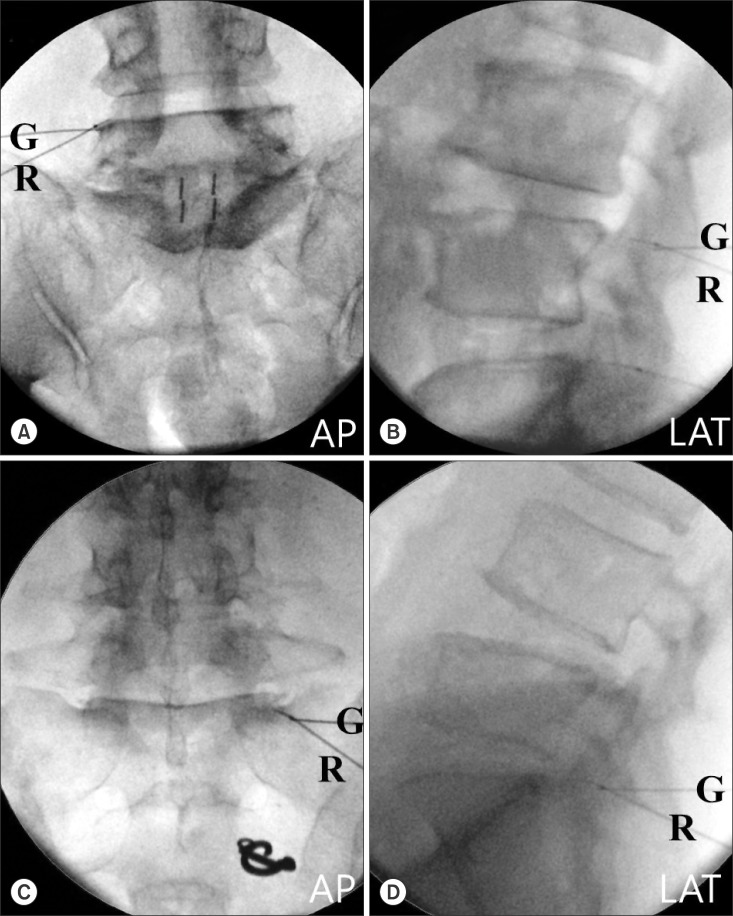
Fig. 3
Duration of RF-MB according to the group by paravertebral muscle twitching. The duration of RF-MB was 4.6, 5.8, and 7.0 months in the None, Partial, and Complete groups, respectively.
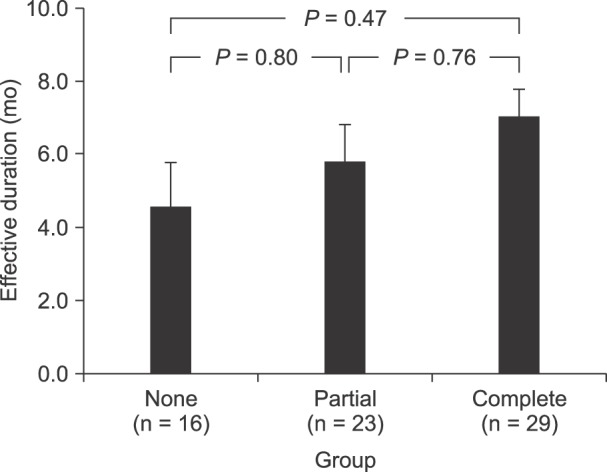
Table 2
Ratio of Paravertebral Twitching to Sensory Provocation in Voltage
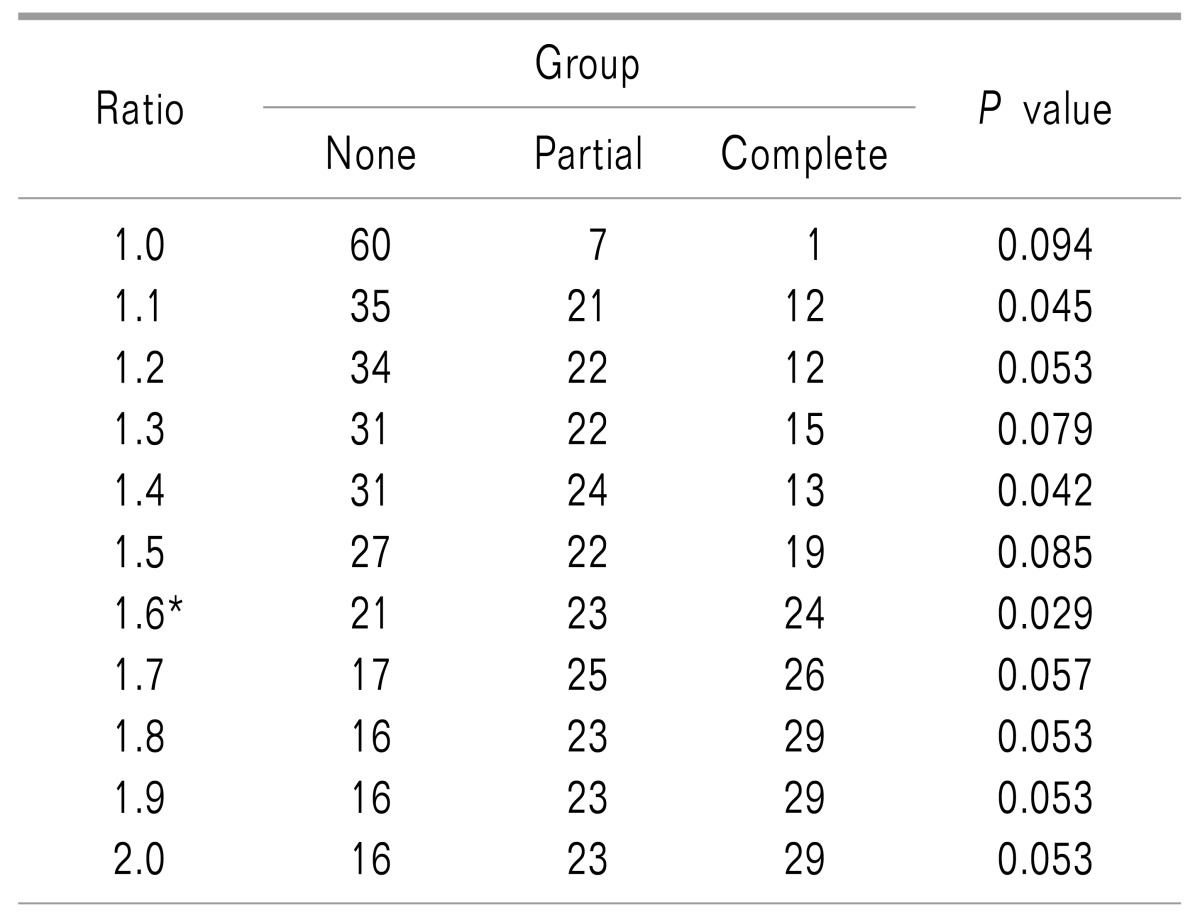
P values were calculated to detect the differences between the groups in terms of the long-term effects (> 6 months) after radiofrequency neurotomy when each multiple of the sensory provocation voltage level was used as a cutoff value for paravertebral muscle twitching. *When the paravertebral muscle twitching was observed within 1.6 times the voltage level at which sensory provocation was achieved, the P value was the lowest.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download