Abstract
Background
To manage intractable cancer pain, an alternative to systemic analgesics is neuraxial analgesia. In long-term treatment, intrathecal administration could provide a more satisfactory pain relief with lower doses of analgesics and fewer side-effects than that of epidural administration. However, implantable drug delivery systems using intrathecal pumps in Korea are very expensive. Considering cost-effectiveness, we performed epidural analgesia as an alternative to intrathecal analgesia.
Methods
We retrospectively investigated the efficacy, side effects, and complications of epidural morphine and local anesthetic administration through epidural catheters connected to a subcutaneous injection port in 29 Korean terminal cancer patients. Patient demographic data, the duration of epidural administration, preoperative numerical pain rating scales (NRS), side effects and complications related to the epidural catheterization and the drugs, and the numerical pain rating scales on the 1st, 3rd, 7th and 30th postoperative days were determined from the medical records.
Results
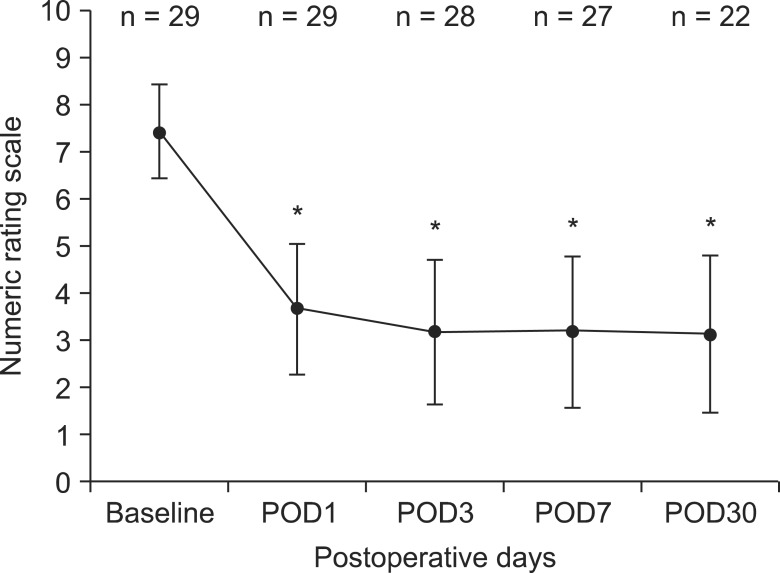
The average score for the numerical pain rating scales for the 29 patients decreased from 7 ± 1.0 at baseline to 3.6 ± 1.4 on postoperative day 1 (P < 0.001). A similar decrease in pain intensity was maintained for 30 days (P < 0.001). Nausea and vomiting were the most frequently reported side effects of the epidural analgesia and two patients (6.9%) experienced paresthesia.
As a consequence of recent developments in palliative therapy for malignant cancers, some patients with limited life expectancy are suffering from severe pain that are uncontrollable with traditional systemic medications. An alternative to systemic analgesics is neuraxial analgesia. Spinal analgesics have been suggested as the fourth step in the World Health Organization guidelines in the management of cancer pain. Despite its invasiveness and risk of infection, spinal analgesics allow more intense and selective analgesia with much lower doses of analgesic agents and less side effects compared to systemic administration. Recent comparative studies have shown that, in long-term treatment, intrathecal administration could provide a more satisfactory pain relief with lower doses of analgesics and fewer side-effects than that of epidural administration [1,2,3,4,5]. However, the implantable drug delivery systems with intrathecal pumps in Korea are very expensive, and cannot be financed by the National Health Insurance. Moreover, although it is a rare case, some reviews on intrathecal catheterization have reported granuloma formation in the subdural space because of highly concentrated morphine [6]. In addition, postdural puncture headache because of cerebrospinal fluid leakage can be an annoying problem in cancer pain patients. Considering the cost effectiveness, epidural analgesia could be an alternative to intrathecal analgesia for managing intractable cancer pain in Korea. However, there are only a few case reports on the use of epidural administration through a subcutaneously connected port in cancer pain patients in the literature published in Korea. We report here our technique used for epidural catheterization and port implantation, the average lifespan of the catheter, and technical complications.
Twenty-nine patients were retrospectively reviewed with malignant cancer pain referred to the pain clinic because of inadequate analgesia with systemic analgesics usually due to their dose-limiting side effects and failure to control pain with palliative procedures including radiation therapy. After obtaining informed consent, we performed epidural catheterization without subcutaneous tunneling and administered morphine and local anesthetics with a patient controlled analgesia (PCA) machine for one week to judge the effectiveness of this therapy and also to titrate the dose of the opioid. From the 24-hour-dose of epidural morphine calculated from the previous systemic dose based on commonly used equianalgesic conversion rules (1 mg epidural morphine = 10 mg IV morphine = 30 mg oral morphine) [7], the hourly dose of morphine could be determined. The calculated dose of morphine was diluted in 0.1% levobupivacaine and administered at a rate of 1 ml/h, and neostigmine 500 ug was added to a total mixture of 200 ml. Therefore each milliliter of the mixture contained an hourly epidural dose of morphine and 2.5 ug of neostigmine. A bolus dose of 2 ml with a 30-min lock-out was also provided. Thereafter, the continuous infusion dose was adjusted according to the pain intensity and bolus dose requirement. When performing the refill of the PCA, the concentration of morphine was modified to deliver its adjusted hourly dose at an infusion rate of 1 ml/hr again. Epidural pain control was discussed with the patient and family members. If family members were willing to accept responsibility for home catheter care and epidural administration, the patient was offered implantation of the epidural catheter and subcutaneous port.
Epidural catheters were placed implanted sterile surgical techniques. All procedures were performed with fluoroscopic guidance under local anesthesia sometimes with intravenous sedation. The skin, slightly medial to the pedicle at the level of one or two segments below from the target interlaminar space, was anesthetized with local anesthetics. The epidural space was accessed with an 18-gauge Tuohy needle with the loss-of-resistance technique. And the catheter was advanced to the targeted segments of the epidural space on the ipsilateral side to cover the affected region. A subcutaneous pocket for the injection port was created caudal to the incision in the lower lateral chest or subclavicular area. Then the epidural catheter was tunneled toward the pocket and connected to the subcutaneous port (Healthport Spinal®, Baxter, USA) (Fig. 1). The catheter and port were connected which was slid along the catheter and outlet tube of the port. Then the connector was tightly screwed onto the threaded part of the outlet tube. After anchoring the injection port to the subcutaneous fascia, the wound was closed. Immediately after completing the implantation procedure, a disposable PCA device (Accufuser plus®, Woo-Young Medical, Korea) was connected to the subcutaneous port through a noncoring needle (Huberlong®, B. Braun, Germany). The regimen consisting of morphine at a predetermined concentration in 0.1% levobupivacaine 250 ml and 0.5 mg of neostigmine for additional analgesic effect was administered at 1 ml/hr and a bolus dose of 1.5 ml with a 15-min lock-out.
Patient demographic data, type of cancer, follow-up period, preoperative and postoperative numerical pain rating scales, epidural opioid-induced side effects, and complications related to epidural catheterization were reviewed from the medical records.
Statistical analyses were performed with SPSS software (version 19.0, SPSS Inc., USA). All the data are expressed as the mean ± SD or number of patients (percentage). All the follow-up data were compared with baseline measures using Student's t-test when assumptions of normality were met or the Wilcoxon signed-ranks test. A significant difference was acknowledged if the probability of a type 1 error was < 5% (i.e., P < 0.05).
Table 1 presents the demographic data of the patients. The follow-up periods ranged from 4 to 394 days. Twenty-seven (93.1%) of the 29 patients died or were transferred to a local hospital for hospice care 37.5 ± 66.19 days after the catheterization, and 2 patients (6.9%) remain alive. Abdomen and lower back pain is the most common pain site and only two patients complained of neck and arm pain (Table 2). Compared with lower body pain, upper body pain was hard to control with epidural catheterization. Any remaining pain was controlled with systemic opioids. Fig. 2 shows the numerical pain rating scales at baseline and at each follow-up. The average score for the numerical pain rating scales for the 29 patients decreased from 7 ± 1.0 at baseline to 3.6 ± 1.4 on postoperative day 1 (P < 0.001). A similar decrease in pain intensity was maintained for 30 days (P < 0.001). Most of the patients had reliable and acceptable pain relief during the epidural analgesia. Seven patients died or were transferred within 30 days.
Table 3 presents the opioid-related side effects and technical complications. Nausea and vomiting were the most frequently reported side effects of epidural morphine administration, and controlled with ondansetron or metoclopramide without reducing epidural opioid dosage. Two patients (6.9%) experienced paresthesia. One complained of right leg numbness and the other had a tingling sensation in the arm. The former maintained the catheter until death, and the latter had the catheter removed and reinserted. In two patients, the epidural catheter was removed because of occlusion. Three patients had injection pain, but they did not need any further treatment because the pain was endurable. No infectious complications from the epidural drug administration through the subcutaneous port were noted. In addition, respiratory depression and urinary retention were absent.
Chronic malignant pain can be effectively treated by epidural administration of the combination of opioid and local anesthetics and other analgesic agents [8]. Compared with bupivacaine, levobupivacaine has a similar clinical profile and a lower potency for motor blockade but an enhanced safety profile about cardiovascular and central nervous system toxicity in regional analgesia [9]. Neostigmine, an acetylcholinesterase inhibitor, increases acetylcholine concentrations which is responsible for the additive analgesic effect in common pain pathways and preganglionic sympathetic neurons [10].
This study showed a significant reduction in the score for the numerical pain rating scales with tolerable pharmacological side effects from epidural morphine and levobupivacaine administration through an epidural catheter connected to a subcutaneous injection port.
Although neuraxial infusion of analgesics can provide adequate pain relief, it could also place a patient at risk of infection. Because the tip of the catheter is in close proximity to the central nervous system, a devastating result such as epidural abscess or meningitis could occur, possibly with neurologic deficits. The possible routes of contamination in an epidural catheter related infection could be colonization of the catheter by skin flora, contamination of the injectate, or hematogenous spread from a distant infectious process [11], although the latter two are much less likely sources of contamination [12]. To minimize the risk of infection, all catheters were placed by experienced anesthesiologists under strict aseptic conditions [13]. In addition, to prevent contamination of the infusate, we used an antimicrobial filter and modern processing techniques [14,15]. Nevertheless, it seems intuitive that the longer the catheter stays in the central nervous system of immunocompromised terminal cancer patients, the greater the risk of serious infection. The infection rate per 1000 catheter-days in patients with implanted catheters with subcutaneous injection ports was half that of percutaneous catheters [16]. Additionally, the infections did not develop in the subcutaneous port systems during much longer time periods after implantation compared with the percutaneous catheters. Moreover, the incidence of catheter dislodgment and kinking was also lower in totally implanted systems than in percutaneous catheters without subcutaneous injection ports [16,17]. These results impy that the use of totally implanted epidural catheters with injection ports could reduce the complication rate in cancer pain patients.
Epidural infusion of local anesthetics are associated with hypotension, sensory loss, proprioceptive changes, and motor weakness [18]. The risk of hypotension is increased in patients with hypovolemia. Inadvertent intrathecal or intravascular injection of epidural doses of local anesthetics have resulted in fatalities [19]. The risks of these events can be reduced by giving a small test dose of local anesthetic with epinephrine to identify improper catheter placement [20]. Nausea and vomiting, respiratory depression, and pruritus are pharmacologic side effects of epidural opioids. These complications are relatively rare in patients who have been chronically exposed to systemic opioids and can be treated by naloxone. The incidence of nausea and vomiting, the most frequently reported opioid related side effects in our study, was only 17.2% and these side effects were well controlled with antiemetics.
The decision to insert an epidural or an intrathecal catheter could be based on several factors such as the patient's life expectancy, the presence of epidural metastasis or impending spinal cord compression, and economical, geographical, or other social environments of the patients [21]. Bedder et al. [22] suggested that after 3 months of treatment, intrathecal analgesia could be more economical than epidural catheters. Crul and Delhaas [4] reported that the intrathecal route is preferred in patients expected to live for more than 1 month because the incidence of complications in the epidural group became higher beyond 20 days of therapy. However, in Korea, the expensive implantable intrathecal drug delivery systems are not covered by the National Health Insurance and other external devices capable of highly delicate infusion for intrathecal delivery are not available. To get an appropriate analgesia, just as with epidural catheters, the tip of the intrathecal catheter needs to be located close to the spinal cord segment of nociception, particularly if a local anesthetic is to be used together [21]. In the case of upper limb or chest wall pain, advancing the catheter rostral to the conus and spinal cistern could increase the risk of serious complications such as traumatic syrinx or transverse myelopathy, and keeping the catheter at that location carries the risk of cord compression because of intrathecal granuloma and prolonged respiratory depression especially after a bolus dose [23,24]. Device infection or drug reservoir contamination could result in meningitis with neurologic complications. In addition, post-implant spinal headache is the most frequently encountered problem in patients with an intrathecal catheter [4]. Therefore, we have chosen the epidural root as a neuraxial administration rather than intrathecal catheterization.
There are several limitations to this study. First, it is a retrospective review of only 29 patients without a control group. The analysis of the efficacy and complication rates drawn from this small sample size could be inadequate. Therefore, this technique needs to be further evaluated with a larger number of subjects and a control group. Second, this technique is not a widely used modality in cancer pain management even for anesthesiologists thus the geographical location of the patients often limits the application of this technique. Most medical institutions in remote area are not equipped with anesthesiologists specializing in pain management and instruments that manage problems with this technique in cancer patients.
Continuous epidural infusion through a port is not a first line of treatment. Other less invasive modalities should be tried first. However, with effective prevention of infection and other possible complications, long-term epidural morphine and local anesthetic infusion with a subcutaneous pump seems to have an acceptable risk-benefit ratio and allows a high degree of autonomy to patients with cancer pain.
References
1. Nordberg G. Epidural versus intrathecal route of opioid administration. Int Anesthesiol Clin. 1986; 24:93–111. PMID: 2872171.

2. Morgan M. The rational use of intrathecal and extradural opioids. Br J Anaesth. 1989; 63:165–188. PMID: 2669907.

3. Nitescu P, Appelgren L, Linder LE, Sjöberg M, Hultman E, Curelaru I. Epidural versus intrathecal morphine-bupivacaine: assessment of consecutive treatments in advanced cancer pain. J Pain Symptom Manage. 1990; 5:18–26. PMID: 2324557.

4. Crul BJ, Delhaas EM. Technical complications during long-term subarachnoid or epidural administration of morphine in terminally ill cancer patients: a review of 140 cases. Reg Anesth. 1991; 16:209–213. PMID: 1911496.
5. Kim JH, Jung JY, Cho MS. Continuous intrathecal morphine administration for cancer pain management using an intrathecal catheter connected to a subcutaneous injection port: a retrospective analysis of 22 terminal cancer patients in Korean population. Korean J Pain. 2013; 26:32–38. PMID: 23342205.

7. Krames ES. Intraspinal opioid therapy for chronic nonmalignant pain: current practice and clinical guidelines. J Pain Symptom Manage. 1996; 11:333–352. PMID: 8935137.

8. Nitescu P, Sjöberg M, Appelgren L, Curelaru I. Complications of intrathecal opioids and bupivacaine in the treatment of "refractory" cancer pain. Clin J Pain. 1995; 11:45–62. PMID: 7540439.

10. Ross VH, Pan PH, Owen MD, Seid MH, Harris L, Clyne B, et al. Neostigmine decreases bupivacaine use by patient-controlled epidural analgesia during labor: a randomized controlled study. Anesth Analg. 2009; 109:524–531. PMID: 19377050.

11. Du Pen SL, Peterson DG, Williams A, Bogosian AJ. Infection during chronic epidural catheterization: diagnosis and treatment. Anesthesiology. 1990; 73:905–909. PMID: 2240680.
12. Smitt PS, Tsafka A, Teng-van de, van der Holt R, Elswijk-de Vries I, Elfrink E, et al. Outcome and complications of epidural analgesia in patients with chronic cancer pain. Cancer. 1998; 83:2015–2022. PMID: 9806662.

13. Maki DG, Ringer M, Alvarado CJ. Prospective randomised trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet. 1991; 338:339–343. PMID: 1677698.

14. De Cicco M, Matovic M, Castellani GT, Basaglia G, Santini G, Del Pup C, et al. Time-dependent efficacy of bacterial filters and infection risk in long-term epidural catheterization. Anesthesiology. 1995; 82:765–771. PMID: 7533485.

15. Waghorn DJ. Intravascular device-associated systemic infections: a 2 year analysis of cases in a district general hospital. J Hosp Infect. 1994; 28:91–101. PMID: 7844353.

16. de Jong PC, Kansen PJ. A comparison of epidural catheters with or without subcutaneous injection ports for treatment of cancer pain. Anesth Analg. 1994; 78:94–100. PMID: 8267188.

17. Carde P, Cosset-Delaigue MF, Laplanche A, Chareau I. Classical external indwelling central venous catheter versus totally implanted venous access systems for chemotherapy administration: a randomized trial in 100 patients with solid tumors. Eur J Cancer Clin Oncol. 1989; 25:939–944. PMID: 2666137.

18. Orser B. Obstetrical epidural anaesthesia in a Canadian outpost hospital. Can J Anaesth. 1988; 35:503–506. PMID: 3168135.

19. Prince G, McGregor D. Obstetric epidural test doses. A reappraisal. Anaesthesia. 1986; 41:1240–1250. PMID: 3544942.
21. de Leon-Casasola OA. Interventional procedures for cancer pain management: when are they indicated? Cancer Invest. 2004; 22:630–642. PMID: 15565820.

22. Bedder MD, Burchiel K, Larson A. Cost analysis of two implantable narcotic delivery systems. J Pain Symptom Manage. 1991; 6:368–373. PMID: 1908884.

23. Harney D, Victor R. Traumatic syrinx after implantation of an intrathecal catheter. Reg Anesth Pain Med. 2004; 29:606–609. PMID: 15635521.

24. Schimpf J, Krakow K, Vehreschild N, Pohlmann-Eden B. Transverse myelopathy as a complication following long-term intrathecal application of morphine in chronic back pain. Anasthesiol Intensivmed Notfallmed Schmerzther. 1999; 34:506–509. PMID: 10494374.

Fig. 2
Numeric rating scale at base-line and at the follow-up visits. The values are expressed as the means ± SD. Significant decreases in numeric rating scale were observed on postoperative days to baseline. *P < 0.05 compared to baseline using the Wilcoxon signed-ranks test.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download