Abstract
Background
The chronic pain can disturb physical, psychological, and social performances. Analgesic agents are widely used but some antidepressants (ADs) showed analgesia also. Bupropion is using for smoke cessation but it can change morphine withdrawal signs such as pain. This study tested the acute systemic effect of bupropion on formalin induced pain behavior in rats.
Methods
Wistar male healthy rats were divided into 7 groups (control, sham, and 5 treated groups with 10, 30, 90, 120, and 200 mg/kg of bupropion, i.p.). The bupropion injected 3 hours prior to formalin induced pain behavior. Formalin (50 µl, 2.5%) was injected subcutaneously in dorsal region of right hindpaw in all animals. Nociceptive signs were observed continuously on-line and off-line each minute. Common pain scoring was used for pain assessment.
Chronic pain is a complicated pain and in the form of resistant to drug therapy, its treatment involved in surgery including rhizotomy, cordotomy, leukotomy, tractotomy, myelotomy, and several other operations [1,2]. The formalin test is using as animal model of human chronic pain, in which the low concentrations of formalin solution (0.5 to 5%) inject into the dorsal (or plantar) region of the hind paw intradermally [3]. The nociceptive behaviors can be measured electrophysiological or by behavioral observation. In the formalin test the classic pain assessing includes four level of the painful behavior (score as 0 to 3), but flinching/shaking or licking/biting of the injected paw are assessed [4,5]. When formalin is applied to animal paws, a two-stage response appeared. A transient early (first) phase followed by a late (second) phase. The first phase is relate to direct peripheral nociceptive afferents but the second phase depends upon prolonged changes in the central nervous system function (central sensitization) [5,6,7,8].
Bupropion is using as smoking cessation agent despite its classification as an AD [9]. The mechanisms of smoke cessation and antidepression by bupropion have not been completely clarified [10]. Bupropion antagonize acetylcholine receptors (nAChRs) mainly, but it can also inhibits synaptic dopamine (DAT) and noradrenalin transporter (NET) [11]. Semenchuk and colleagues [12] found that 73% of subjects with neuropathic pain studied in their placebo-controlled trial obtained pain relief with bupropion treatment. El Mansari et al. [13] showed that sustained administration of the bupropion alters the neuronal activity of serotonin (5-HT), norepinephrine (NE) but not dopamine (DA) neurons in the rat brain. Bupropion has not effect on low back pain with non-neuropathic origin [14] but it reliefs neuropathic pain in human cases [12,15,16].
Our recent studies have showed that the injection of the bupropion in the ventral tegmental area (VTA) can alter occurrence and pattern of the pain and opioid withdrawal behaviors [17]. Intra-VTA bupropion also can alter the aggressive behaviors in non dose dependent manner [18]. The intra locus coeruleus (LC) microinfusion of the bupropion abolishes first and second phase significantly [19]. The present study designed to evaluate the effect of intraperitoneal injection of the bupropion on formalin induced pain behaviors in male healthy rats.
All experiment procedures and protocols were in accordance with the guidelines for the Care and Use of Experimental Animals outlined by the Laboratory Animal Center of Urmia University of Medical Sciences. All procedures and protocols were approved by the Urmia Medical Science Research Ethics Committee (UMSREC) and performed in accordance with the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals. The animals housed in groups of 3 per cage. Animals were housed at 12 h light/dark cycle (7:00 am-7:00 pm) and controlled temperature (22 ± 2℃) with food and water ad libitum. The pain assessment carried out after acclimation (7 days).
Wistar healthy male adult rats (Pasteur Institute, Tehran-Iran, weighing 200-250 g) divided into 7 groups (n=6 in each group): control, sham and 5 groups with injection of bupropion (10, 30, 90, 120, and 200 mg/kg, i.p.). The control group had no drug injection and sham group had sterile normal saline (in the same volume of treated rats, i.p.) as drug vehicle. All rats received formalin (2.5%, 50 µl, freshly prepared) in dorsal surface of right hind paws intradermally. The peak plasma concentration of bupropion (hydroxybupropion) is achieved 3 hours after administration and for this reason the bupropion injected in treated groups 3 hours before formalin test [20].
After the acclimation (30 min) each rat received 50 µl of formalin solution (2.5% in normal saline) subcutaneously into the dorsal surface of right hindpaw using a microsyringe with a 26-gauge needle. In an open Plexiglas observation chamber (30 × 30 × 30 cm) the pain score of the injected paw was acquisitioned on-line by an observer and recorded by 3 digital cameras for off-line analysis. A mirror was placed at an angle of 45° under the transparent floor to clear observation. The Dubuisson and Dennis [4] procedure of pain rating method was applied. Four nociceptive scores registered each minute up to 90 min. Briefly in this method the scores were: 0 = normal behavior of the hind limbs to support the body; 1 = slight touching of the injected paw on the glass surface to lightly support or not support the body; 2 = total withdrawal of the injected paw; and 3 = licking, biting or shaking of the injected paw [4]. In this research the data between 0 to 10 min after formalin injection represented as phase one (early phase) and between 16 to 90 min represented as phase two (late phase). In this study the pain score was recorded and expressed in a 5 min time blocks. All animals were used only once and experiments were carried out in the same day time (11.00 to 13.00 h). The off-line pain analysis is carried out by 2 another observer with blind approach.
Drugs and chemicals are used in the present study included; Bupropion, Formalin (Sigma-Aldrich, USA), Sterile normal saline (SUPA, Iran), Hamilton microsyringes (Hamilton Bonaduz AG, Switzerland).
The data were analyzed by one-way repeated measure analysis of variance (ANOVA) followed by the post-hoc Tukey's test. The statistical significance was P < 0.05. Results are expressed as mean ± SEM. The GB-Stat ver. 5.0 statistical software was used for statistical analysis and Microsoft Excel ver. 2003 was used for graphical presentation of the data.
Two spontaneous nociceptive behaviors recorded following subcutaneous injection of formalin in the right hindpaw: (1) pain score of the paw, and (2) licking/biting duration of injected paw. The two induced nociceptive behavior showed a biphasic pattern. The first phase (minutes 0 to 10) and second phase (minutes 16-90) is shown in the figures.
Fig. 1A shows the formalin induced pain scores in a time line of 90 minutes in a 5 min time blocks. The control, sham, and 10 mg/kg of bupropion treated groups had no significant difference in the pain scores but the 30, 90, 120, and 200 mg/kg of bupropion treated groups showed the significant and dose dependent decrease in the pain scores.
Fig. 1B shows the cumulative formalin induced pain scores in the first phase and second phase in all groups. There was no significant difference in cumulative pain scores in the first phase. In the second phase the data of control, sham, and 10 mg/kg of bupropion groups had no significant difference but the cumulative pain scores in the second phase showed the significant decrease in the doses of 30, 90, 120, and 200 mg/kg of bupropion dose dependently. The dose increasing showed greater decrease in the pain scores.
Fig. 2A shows the formalin induced licking/biting duration in a time line of 90 minutes in a 5 min time blocks. The control, sham, 10, and 30 mg/kg bupropion treated groups had no significant difference in the licking/biting duration but the 90, 120, and 200 mg/kg of bupropion treated groups showed the significant and dose dependent decrease in the licking/biting duration.
Fig. 2B shows the cumulative formalin induced licking/biting duration in the two phases of the formalin test in all groups. In the first phase there was no significant difference in data of control, sham, 10, and 30 mg/kg of bupropion groups. In the second phase all treated groups with bupropion showed the significant decrease in the licking/biting duration.
In summary the intraperitoneal application of bupropion can decrease the formalin induced pain behavior dose dependently. The elevation of dose of bupropion can inhibits pain behavior more than low doses. The pain behavior in the second phase decreased greater than of the first phase one.
The well documented mechanisms about the antidepression effects of ADs is the inhibition of the reuptake of neurotransmitters such as DA, NE, and 5-HT or inhibition of the catabolism of neurotransmitters [21,22]. The analgesic effect of ADs have well studied and reviewed previously [23]. The mechanisms of ADs for pain killing have not been well known yet. Serotonin, DA, and NE reuptake inhibition is the common mechanism for anti-nociception actions of ADs with different efficacy and sensitivity. The anti-nociception doses of ADs differ from anti-depression [24,25,26]. Therefore, the most common mechanism of the effect of bupropion is inhibition of NE and also DA reuptake for anti-nociception. Along with bupropion some other ADs have the anti-nociception effect also [27,28,29].
According to the present study, the systemic bupropion had the analgesic effects on the formalin induced pain behaviors. It seems that this effects can achieved by NE reuptake inhibition and increase the NE in the noradrenergic synapses. This effect can elevate the NE for a transient period. It seemed that the elevation of NE for analgesia is longer that than achieved by NE reuptake inhibition. The LC is the main noradrenergic nucleus of the brain and is a major target for bupropion and other analgesic agents. The presence and elevation of bupropion in the vicinity of LC neurons increase the NE in the dendritic synapses of LC but this elevation can inhibit the LC neuronal firing rate due to autoreceptor inhibition. This is the paradox effect but recent studies of neuronal activity of LC nucleus in adjacent some of ADs revealed that, they can decrease the firing rate of LC neurons despite their anti-nociceptive effects [30,31]. In contrast to anti-nociception, the LC lesions can reduce tonic behavioral responses to intraplantar formalin injection [32,33]. Many researches concluded that LC neuronal firing rate can increase in pain modulation via neuronal pathway to inhibition of central pain nuclei [34,35,36]. The effect of sustained bupropion administration (s.c.) produce a dose-dependent attenuation of the mean spontaneous firing of LC neurons (7.5 mg/kg per day: 15%; 15 mg/kg per day: 61%; 30 mg/kg per day: 80%). This attenuation is reversed by the alpha 2-adrenoceptor antagonist idazoxan. Sustained bupropion administration decreased the firing rate of NE neurons due to an increase in activation of their inhibitory somatodendritic alpha 2-adrenoceptors. This effect of the bupropion treatment would be attributable mainly to an enhancement of NE release and not to reuptake inhibition [37].
Although several researches have showed that the neuronal firing rate of LC neurons enhances in acute pain (tail pinch, footshock, heat) [38,39,40] and also chronic pain [41,42,43] but Alba-Delgado and co-workers have showed that the LC neuronal firing rate did not change in Chronic Constriction Injury (CCI) as a model of neuropathic pain [44]. On the other hand, NE reuptake inhibitor desipramine, the elevated endogenous NE attenuate the firing rate of LC neurons [30]. Rosenberg et al. [45] studied the pain-stimulated and pain-depressed behaviors of the various ADs. The results of this study suggest that the using of some DA/NE/5-HT reuptake inhibitors as anti-nociceptive agents in some circumstances.
The projection of the LC to spinal cord (descending pain pathway), exerts inhibitory influences on pain threshold. Furthermore, projections from LC nucleus to spinal cord control the release of the 5-HT and NE at the level of the spinal cord. As a general rule, when these monoamines increased in synaptic cleft within the spinal cord, it makes a decrease in the pain threshold. However, it should be noted that 5-HT can both dampen and enhance the sensation of pain, depending on the receptor subtypes activated. On the other hand the ADs are the most effective treatment to deal with chronic pain of diverse origins, with or without co-existing depression [46,47]. At the supraspinal level, these compounds increase NE and 5-HT levels in the synapses while simultaneously enhancing the activity of the descending inhibitory bulbospinal pathways, thereby producing analgesia.
The results of this study suggest that the acute systemic injection of bupropion can alleviate pain behaviors significantly. The formalin induced pain behavior of second phase is prominent decreased than first phase. It seems that the bupropion acts on the central pain modulation. Our recent study about the direct effect of the bupropion on the LC neurons showed that formalin induced pain is reduced by intra-LC microinfusion (1 µl/5 min in each side via previous cannulation) of bupropion [19]. Our other studies about the effect of microinfusion of bupropion into the ventral tegmental area revealed that the gama-aminobutyric acid (GABA) releasing neurons is inhibited strongly by bupropion and remove the inhibition (disinhibition) of the VTA-DA neurons excite them [48]. We proposed that the same mechanism occurs in the LC and activate some LC-NE neurons to achieve the analgesic effects of the bupropion.
The bupropion has analgesic effect in the systemic application. Bupropion decreased the second phase of pain behavior of the formalin test dominantly. Bupropion had less effect on first phase than second phase of formalin induced pain behavior. It concluded that the analgesic effects of bupropion are more related to central nervous system mechanisms. Evidence of mechanism of analgesic effects of bupropion is limited and needs to other studies.
ACKNOWLEDGEMENTS
This research supported by the Research and Technology vice chancellor of the Urmia University of Medical Sciences (grant No. 1010) and conducted in the Danesh Pey Hadi Co., Faculty of Medicine, Urmia University of Medical Sciences.
References
1. Kang SS, Jung JW, Song CK, Yoon YJ, Shin KM. A new anterior approach for fluoroscopy-guided suprascapular nerve block - a preliminary report -. Korean J Pain. 2012; 25:168–172. PMID: 22787547.

2. Park HJ, Moon DE. Pharmacologic management of chronic pain. Korean J Pain. 2010; 23:99–108. PMID: 20556211.

3. Le Bars D, Gozariu M, Cadden SW. Acute pain measurement in animals. Part 1. Ann Fr Anesth Reanim. 2001; 20:347–365. PMID: 11392245.
4. Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977; 4:161–174. PMID: 564014.

5. Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992; 51:5–17. PMID: 1454405.

6. Vaccarino AL, Chorney DA. Descending modulation of central neural plasticity in the formalin pain test. Brain Res. 1994; 666:104–108. PMID: 7889357.

7. Lebrun P, Manil J, Colin F. Formalin-induced central sensitization in the rat: somatosensory evoked potential data. Neurosci Lett. 2000; 283:113–116. PMID: 10739888.

8. Abbadie C, Taylor BK, Peterson MA, Basbaum AI. Differential contribution of the two phases of the formalin test to the pattern of c-fos expression in the rat spinal cord: studies with remifentanil and lidocaine. Pain. 1997; 69:101–110. PMID: 9060019.

9. Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995; 56:395–401. PMID: 7665537.
10. Clayton AH. Extended-release bupropion: an antidepressant with a broad spectrum of therapeutic activity? Expert Opin Pharmacother. 2007; 8:457–466. PMID: 17309340.

11. Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev. 2006; 12:178–207. PMID: 17227286.

12. Semenchuk MR, Sherman S, Davis B. Double-blind, randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology. 2001; 57:1583–1588. PMID: 11706096.

13. El Mansari M, Ghanbari R, Janssen S, Blier P. Sustained administration of bupropion alters the neuronal activity of serotonin, norepinephrine but not dopamine neurons in the rat brain. Neuropharmacology. 2008; 55:1191–1198. PMID: 18708076.

14. Katz J, Pennella-Vaughan J, Hetzel RD, Kanazi GE, Dworkin RH. A randomized, placebo-controlled trial of bupropion sustained release in chronic low back pain. J Pain. 2005; 6:656–661. PMID: 16202958.

15. Semenchuk MR, Davis B. Efficacy of sustained-release bupropion in neuropathic pain: an open-label study. Clin J Pain. 2000; 16:6–11. PMID: 10741812.

16. Shah TH, Moradimehr A. Bupropion for the treatment of neuropathic pain. Am J Hosp Palliat Care. 2010; 27:333–336. PMID: 20185402.
17. Ghaderi Pakdel F, Naderi S, Zare S. The opposite effect of intra-VTA bupropion on chewing and escape attendance behaviors of morphine withdrawal syndrome in rat. Urmia Med J. 2011; 21:415–422.
18. Mokhtari Hashtjin M, Zare S, Ghaderi Pakdel F, Heysieattalab S. The effect of intra-VTA injection of Bupropion on submissive defensive aggressive behavior induced by electrical foot shock of rat. Pharm Sci. 2010; 16:125–130.
19. Jahanbani M, Nasri S, Ghaderi Pakdel F, Cankurt U, Shahabi P, Amirabadi S, et al. The effect of acute intra Locus Coeruleus (LC) microinfusion of bupropion on formalin induced pain behavior in rat. Basic Clin Neurosci. 2014; 5:31–41.
20. Fava M, Rush AJ, Thase ME, Clayton A, Stahl SM, Pradko JF, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005; 7:106–113. PMID: 16027765.
21. Randrup A, Braestrup C. Uptake inhibition of biogenic amines by newer antidepressant drugs: relevance to the dopamine hypothesis of depression. Psychopharmacology (Berl). 1977; 53:309–314. PMID: 408861.

22. Sampson D, Willner P, Muscat R. Reversal of antidepressant action by dopamine antagonists in an animal model of depression. Psychopharmacology (Berl). 1991; 104:491–495. PMID: 1838201.

23. Thaler KJ, Morgan LC, Van Noord M, Gaynes BN, Hansen RA, Lux LJ, et al. Comparative effectiveness of second-generation antidepressants for accompanying anxiety, insomnia, and pain in depressed patients: a systematic review. Depress Anxiety. 2012; 29:495–505. PMID: 22553134.

24. Gallagher RM. Management of neuropathic pain: translating mechanistic advances and evidence-based research into clinical practice. Clin J Pain. 2006; 22:S2–S8. PMID: 16344609.
25. Jackson KC 2nd, St Onge EL. Antidepressant pharmacotherapy: considerations for the pain clinician. Pain Pract. 2003; 3:135–143. PMID: 17163912.

26. Sharp J, Keefe B. Psychiatry in chronic pain: a review and update. Curr Psychiatry Rep. 2005; 7:213–219. PMID: 15935136.

27. Hawley P. Nontricyclic antidepressants for neuropathic pain #187. J Palliat Med. 2009; 12:476–477. PMID: 19416046.

28. Sansone RA, Sansone LA. Pain, pain, go away: antidepressants and pain management. Psychiatry (Edgmont). 2008; 5:16–19. PMID: 19724772.
29. Miller A, Rabe-Jabłońska J. The effectiveness of antidepressants in the treatment of chronic non-cancer pain--a review. Psychiatr Pol. 2005; 39:21–32. PMID: 15771151.
30. West CH, Ritchie JC, Boss-Williams KA, Weiss JM. Antidepressant drugs with differing pharmacological actions decrease activity of locus coeruleus neurons. Int J Neuropsychopharmacol. 2009; 12:627–641. PMID: 18950545.

31. Grant MM, Weiss JM. Effects of chronic antidepressant drug administration and electroconvulsive shock on locus coeruleus electrophysiologic activity. Biol Psychiatry. 2001; 49:117–129. PMID: 11164758.

32. Martin WJ, Gupta NK, Loo CM, Rohde DS, Basbaum AI. Differential effects of neurotoxic destruction of descending noradrenergic pathways on acute and persistent nociceptive processing. Pain. 1999; 80:57–65. PMID: 10204718.

33. Taylor BK, Roderick RE, Basbaum AI. Brainstem noradrenergic control of nociception is abnormal in the spontaneously hypertensive rat. Neurosci Lett. 2000; 291:139–142. PMID: 10984626.

34. Tsuruoka M, Matsutani K, Maeda M, Inoue T. Coeruleotrigeminal inhibition of nociceptive processing in the rat trigeminal subnucleus caudalis. Brain Res. 2003; 993:146–153. PMID: 14642840.

35. Tsuruoka M, Arai YC, Nomura H, Matsutani K, Willis WD. Unilateral hindpaw inflammation induces bilateral activation of the locus coeruleus and the nucleus subcoeruleus in the rat. Brain Res Bull. 2003; 61:117–123. PMID: 12831996.

36. Liu L, Tsuruoka M, Maeda M, Hayashi B, Inoue T. Coeruleospinal inhibition of visceral nociceptive processing in the rat spinal cord. Neurosci Lett. 2007; 426:139–144. PMID: 17913360.

37. Dong J, Blier P. Modification of norepinephrine and serotonin, but not dopamine, neuron firing by sustained bupropion treatment. Psychopharmacology (Berl). 2001; 155:52–57. PMID: 11374336.

38. Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981; 1:887–900. PMID: 7346593.

39. Cedarbaum JM, Aghajanian GK. Activation of locus coeruleus neurons by peripheral stimuli: modulation by a collateral inhibitory mechanism. Life Sci. 1978; 23:1383–1392. PMID: 214648.

40. Hajós M, Engberg G, Elam M. Reduced responsiveness of locus coeruleus neurons to cutaneous thermal stimuli in capsaicin-treated rats. Neurosci Lett. 1986; 70:382–387. PMID: 3022199.

41. Chapman V, Suzuki R, Dickenson AH. Electrophysiological characterization of spinal neuronal response properties in anaesthetized rats after ligation of spinal nerves L5-L6. J Physiol. 1998; 507:881–894. PMID: 9508847.

42. Pertovaara A, Kontinen VK, Kalso EA. Chronic spinal nerve ligation induces changes in response characteristics of nociceptive spinal dorsal horn neurons and in their descending regulation originating in the periaqueductal gray in the rat. Exp Neurol. 1997; 147:428–436. PMID: 9344567.

43. Viisanen H, Pertovaara A. Influence of peripheral nerve injury on response properties of locus coeruleus neurons and coeruleospinal antinociception in the rat. Neuroscience. 2007; 146:1785–1794. PMID: 17445989.

44. Alba-Delgado C, Borges G, Sánchez-Blázquez P, Ortega JE, Horrillo I, Mico JA, et al. The function of alpha-2-adrenoceptors in the rat locus coeruleus is preserved in the chronic constriction injury model of neuropathic pain. Psychopharmacology (Berl). 2012; 221:53–65. PMID: 22038538.

45. Rosenberg MB, Carroll FI, Negus SS. Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. J Pain. 2013; 14:246–259. PMID: 23332494.

46. Mico JA, Berrocoso E, Ortega-Alvaro A, Gibert-Rahola J, Rojas-Corrales MO. The role of 5-HT1A receptors in research strategy for extensive pain treatment. Curr Top Med Chem. 2006; 6:1997–2003. PMID: 17017970.

47. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry. 2003; 54:399–409. PMID: 12893114.

48. Amirabadi S, Ghaderi Pakdel F, Shahabi P, Naderi S. Microinfusion of bupropion inhibits putative GABAergic ventral tegmental area neuronal activity. Basic Clin Neurosci. 2014; [in press].
Fig. 1
(A) The formalin induced pain scores in rats. The all doses of bupropion injected 3 hours before formalin test. Each point represents the mean ± SEM (n = 6) number of pain scores during 5 min observation period. There was no significant difference between control, sham, and 10 mg/kg of bupropion groups. There was significant difference between, sham and doses of 30, 90, 120, and 200 mg/kg of bupropion groups. (B) The cumulative formalin induced pain score in rats. The all doses of bupropion injected 3 hours before formalin test. Cumulative formalin induced pain scores of first phase between all groups had no significant difference but the second phase had significant difference in doses of 30, 90, 120, and 200 mg/kg of bupropion groups in comparison with sham group (one-way repeated measured ANOVA, Tukey's post hoc test, ***P < 0.001, **P < 0.01, and *P < 0.05).
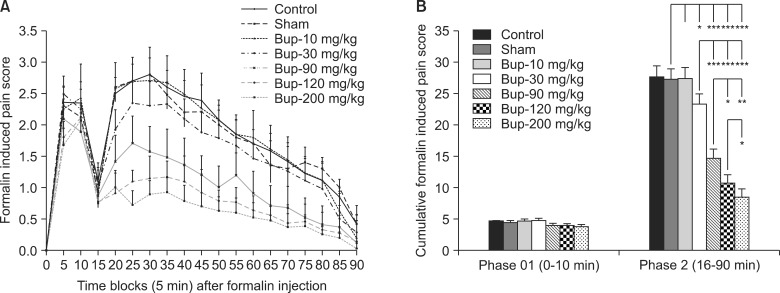
Fig. 2
(A) The formalin induced licking/biting duration in rats. The all doses of bupropion injected 3 hours before formalin test. Each point represents the mean ± SEM (n = 6) number of licking/biting duration during 5 min observation period. There was no significant difference between control, sham, 10, and 30 mg/kg of bupropion groups. There was significant difference between, sham and doses of 90, 120, and 200 mg/kg of bupropion groups. (B) The cumulative formalin induced licking/biting duration in rats. The all doses of bupropion injected 3 hours before formalin test. Cumulative formalin induced licking/biting of phase 01 between groups had no significant difference in groups control, sham, 10, and 30 mg/kg of bupropion. In the phase 02 there is a significant difference between sham and all bupropion treated groups (one-way repeated measured ANOVA, Tukey's post hoc test, ***P < 0.001, **P < 0.01, and *P < 0.05).





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download