Abstract
Conventional transcrural CPB via the "walking off" the vertebra technique may injure vital organs while attempting to proximally spread injectate around the celiac plexus. Therefore, we attempted the CT-simulated fluoroscopy-guided transdiscal approach to carry out transcrural CPB in a safer manner, spreading the injectate more completely and closely within the celiac plexus area. A 54-year-old male patient with pancreatic cancer suffered from severe epigastric pain. The conventional transcrural approach was simulated, but the needle pathway was impeded by the kidney on the right side and by the aorta on the left side. After simulating the transdiscal pathway through the T11-12 intervertebral disc, we predetermined the optimal insertion point (3.6 cm from the midline), insertion angle (18 degrees), and advancement plane, as well as the proper depth. With the transdiscal approach, we successfully performed transcrural CPB within a narrow angle, and the bilateral approach was not necessary as we were able to achieve the bilateral spread of the injectate with the single approach.
Celiac plexus block (CPB) is a frequently used procedure with demonstrated efficacy for patients with abdominal pain originating from visceral pain, especially in chronic pancreatitis and pancreatic cancer patients [1]. The celiac plexus surrounds the celiac trunk and is located deep in the retroperitoneum at the level of the T12 and L1 vertebra, within the vicinity of vital organs such as the liver, kidney, and major vessels [2-5]. Therefore, CPB poses technical challenges such as locating the correct needle position because of several obstacles that are in the pathway of the needle. Many different block techniques have been conducted clinically; these include fluoroscopy- or computed tomography (CT)-guided percutaneous retrocrural, transcrural, or transaortic approaches as well as gastric endoscopic approaches [2,3,6].
The goal of CPB is to achieve better analgesia by trying to locate the proper needle position to improve the spread of the injectate to the celiac plexus area [7]. Conventional transcrural CPB via the "walking off" the vertebra technique may promote the proper spread of the injectate around the celiac plexus, but major organ injury could occur. Therefore, we attempted the CT-simulated transdiscal approach to perform transcrural CPB in a safer manner, spreading the injectate more completely and closely within the celiac plexus area. With the transdiscal approach, we were able to perform CPB within a narrow angle, and the bilateral approach was not necessary because we achieved the bilateral spread of the injectate with the single approach.
A 54-year-old male patient was diagnosed with pancreatic cancer and suffered from intractable, severe epigastric pain with a score of 5 to 8 out of 10 on the Numeric Rating Scale (NRS). Furthermore, the abdominal pain was not responsive to opioids and other medications.
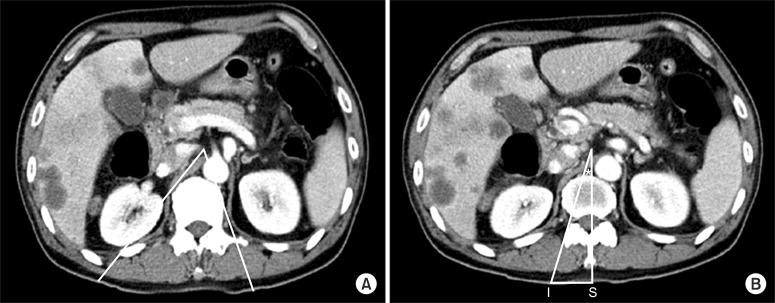
Therefore, we planned to perform celiac plexus neurolysis and reviewed the patient's anatomy on the abdominal CT image with contrast to determine the target point, ideal depth, and insertion angle through CT simulation. The celiac trunk was located at the T12 vertebral body level. At this level, the classic conventional transcrural approach via "walking off" the vertebral body was simulated, but the needle pathway was impeded by the kidney on the right side and by the aorta on the left side (Fig. 1A). Then, we simulated the transdiscal pathway through the T11-12 intervertebral disc. The pathway was drawn from the target point to the lateral side of the right superior articular process of the T12 vertebra and extended to the skin.
The point at which the pathway crossed the posterior skin surface was designated as the needle insertion point (I). The distance from the midline spinous process of the T12 (S) to the needle insertion point was measured against the scale printed on the axial CT image (IS: 3.6 cm). The angle between the proposed needle pathway and the midline was designated as the needle insertion angle (*) (Fig. 1B). The distance between the target point and the anterior margin of the vertebral body was measured, and the anteroposterior (AP) diameter of the vertebral body was also measured. The proportion of these two values was used to estimate the proper depth of the needle in the fluoroscopic image.
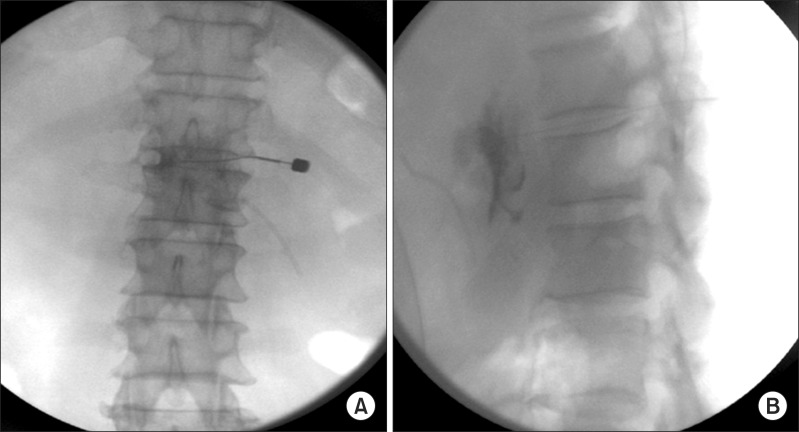
In the operating room, the patient was placed in a prone position, and we identified the T11-12 intervertebral disc space. In this case, the predetermined insertion angle was 18 degrees on the right side. Therefore, we rotated the fluoroscopy tube 18 degrees to the right side. The insertion point was the lateral margin of the superior articular process of the T12, and it was marked 3.6 cm to the right of the midline. After anesthetic infiltration was deepened in a fan fashion, the skin was punctured with a 16 g needle, and a 20 g Chiba needle was introduced through the 16 g needle. After contacting the disc, the fluoroscope was rotated to the lateral position. We inserted the needle through the disc while checking the tip position with the AP and lateral fluoroscopic images. After penetrating the disc, the 20 g Chiba needle was advanced up to the predetermined depth, which was 2/3 of the AP diameter of the vertebral body from the anterior margin of the vertebral body. Frequent fluoroscopic images for both the AP and lateral views were used to guide the needle when advancing it in the correct plane. While advancing the needle, we checked the loss of resistance using saline to penetrate the crura of the diaphragm. Contrast was injected to confirm the proper spread; the contrast flow showed a smooth curvilinear contour corresponding to the anterolateralaortic space, and it silhouetted the runoff of the celiac artery. On the AP view, the contrast spread across the midline. On the lateral view, the needle penetrated the disc, and its tip was located at 2/3 of the AP diameter anterior to the vertebral body. Then, 15 ml of 2% lidocaine followed by 15 ml of 99% ethyl alcohol was injected for neurolysis (Fig. 2).
There were no adverse events during the procedure. The patient tolerated the entire procedure well and did not complain of pain related to the procedure. The next day, during follow-up, the patient reported an abdominal pain score of 2 to 3 out of 10 on the NRS. After 1 week, the patient expressed great satisfaction with the results and was discharged.
Celiac plexus block has been used to relieve abdominal pain originating from various visceral organs. It is known that fluoroscopy- or CT-guided CPB is useful for assuring proper placement of the needle, and several approaches to effectively block the celiac plexus have been described [8]. Among these approaches, fluoroscopy-guided CPB is relatively cheaper and easier to perform compared to CT-guided or endoscopy-guided procedures. Under the fluoroscopy-guided percutaneous posterior approach, one can choose between the transcrural, retrocrural, and transaortic approaches. The transcrural approach is a more difficult method than the retrocrural approach, but the proximity to the celiac plexus may promote the proper spread of the injectate around the celiac plexus. However, the transcrural approach is not always feasible because of several anatomical considerations. The abdominal aorta frequently obstructs the passage needed for the left-sided approach, while the kidney impedes proper needle positioning for the right-sided approach. In such cases, the retrocrural approach can replace the transcrural approach, but there are too few reports comparing the efficacy of these two approaches [9,10].
The conventional transcrural approach, which involves the "walking off" the vertebra technique, has some disadvantages. Needle contact with the bone is painful, and multiple needle redirections are required. Moreover, it is performed bilaterally because the tip of the needle is usually located far from the midline to avoid penetrating organs such as the aorta or kidney [3]. Patients who receive CPB usually complain of severe abdominal pain and may not be able to tolerate the prone position during the procedure, as abdominal compression worsens the abdominal pain. In that case, CPB should be performed in the lateral position, and the bilateral needle approach may be more difficult and time-consuming compared to the unilateral approach. In addition, in the conventional transcrural approach, it is generally suggested that the needle insertion angle start at 45 degrees from the skin surface and be redirected steeply [9]. A wide angle may enable a unilateral block near the midline, but it can increase the risk of organ injury. Yang et al. [11] described the structures that impede the needle pathway via CT simulation. In CPB, needles "walking off" the vertebra within a fixed distance from the midline on both sides frequently injure vital organs. It is easy to use a wide insertion angle to locate the needle near the celiac plexus, but this increases the chance of organ penetration, and a narrower insertion angle may lower the incidence of injury to vital organs or vessels.
To properly place the needle transcrurally in proximity to the celiac plexus, it is necessary to determine the optimal needle insertion point, insertion angle, and advancement plane, as well as the proper depth [12].
CT simulation before the procedure can help avoid organ injury by improving understanding of the anatomy of individual patients, allowing determination of the proper depth and the optimal insertion point, angle, and advancement plane. Fluoroscopy can dynamically monitor needle advancement and contrast spread through continuous imaging. Thus, we took advantage of both imaging techniques by analyzing and measuring the patient's specific anatomy in the celiac trunk region on the CT simulation before the procedure. With this method, we performed the transcrural approach via the transdiscal CPB approach to lower the risk of major organ penetration and reduce the procedural time, while also achieving a narrow insertion angle and unilateral approach.
Penetration of the disc raises concerns about complications related to the disc, such as discitis and disc degeneration. Previous reports on the transdiscal approach for splanchnic nerve block and retrocrural celiac plexus block showed that the transdiscal approach does not add complications related to disc penetration such as discitis or back pain [13]. To reduce the possible complications related to disc penetration, we used careful aseptic techniques such as a sterile drape with povidone-iodine and chlorhexidine and a needle-through-needle technique (16 g and 20 g Chiba needle), and we did not experience any disc-related complications after the transdiscal approach.
In conclusion, we recommend the CT-simulated fluoroscopy-guided transdiscal approach in transcrural CPB instead of the conventional approach if there is the possibility of kidney, liver, lung, or major vessel injury. The suggested approach improves needle placement proximal to the celiac plexus area, increasing patient tolerance and reducing the possibility of organ injury. Further studies should be done to compare the recommended approach with the conventional approach in a larger series.
References
1. Bahn BM, Erdek MA. Celiac plexus block and neurolysis for pancreatic cancer. Curr Pain Headache Rep. 2013; 17:310. PMID: 23299904.

2. Kim WH, Lee CJ, Sim WS, Shin BS, Ahn HJ, Lim HY. Anatomical analysis of computed tomography images for determining the optimal oblique fluoroscope angle for percutaneous coeliac plexus block. J Int Med Res. 2011; 39:1798–1807. PMID: 22117980.

3. Moore DC, Bush WH, Burnett LL. Celiac plexus block: a roentgenographic, anatomic study of technique and spread of solution in patients and corpses. Anesth Analg. 1981; 60:369–379. PMID: 7195158.
4. Ischia S, Luzzani A, Ischia A, Faggion S. A new approach to the neurolytic block of the coeliac plexus: the transaortic technique. Pain. 1983; 16:333–341. PMID: 6194498.

5. Ischia S, Ischia A, Polati E, Finco G. Three posterior percutaneous celiac plexus block techniques. A prospective, randomized study in 61 patients with pancreatic cancer pain. Anesthesiology. 1992; 76:534–540. PMID: 1550278.

6. Garcia-Eroles X, Mayoral V, Montero A, Serra J, Porta J. Celiac plexus block: a new technique using the left lateral approach. Clin J Pain. 2007; 23:635–637. PMID: 17710015.

7. De Cicco M, Matovic M, Bortolussi R, Coran F, Fantin D, Fabiani F, et al. Celiac plexus block: injectate spread and pain relief in patients with regional anatomic distortions. Anesthesiology. 2001; 94:561–565. PMID: 11379673.
8. Loukas M, Klaassen Z, Merbs W, Tubbs RS, Gielecki J, Zurada A. A review of the thoracic splanchnic nerves and celiac ganglia. Clin Anat. 2010; 23:512–522. PMID: 20235178.

9. Hilgier M, Rykowski JJ. One needle transcrural celiac plexus block. Single shot or continuous technique, or both. Reg Anesth. 1994; 19:277–283. PMID: 7947429.
10. Noble M, Gress FG. Techniques and results of neurolysis for chronic pancreatitis and pancreatic cancer pain. Curr Gastroenterol Rep. 2006; 8:99–103. PMID: 16533471.

11. Yang IY, Oraee S, Viejo C, Stern H. Computed tomography celiac trunk topography relating to celiac plexus block. Reg Anesth Pain Med. 2011; 36:21–25. PMID: 21455084.

12. Yang IY, Oraee S. A modified approach to transcrural celiac plexus block. Reg Anesth Pain Med. 2005; 30:303–307. PMID: 15898036.

13. Flanagan MN, Chung BU. Roentgenographic changes in 188 patients 10-20 years after discography and chemonucleolysis. Spine (Phila Pa 1976). 1986; 11:444–448. PMID: 3750081.

Fig. 1
The axial image of abdominal CT at the level T12 showing the celiac trunk. (A) The lines drawn according to the conventional transcrural approach was impeded by kidney in right side and aorta in left side. (B) Right needle pathway via transdiscal in transcrural approach was drawn on the image at the level T12-L1 intervertebra disc. I: the right needle insertion point, S: midline of spinous process, IS: 3.6 cm, *The angle for needle insertion on the right.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download