Abstract
Background
Vincristine-induced peripheral neuropathy is a major dose limiting side effect and thus effective therapeutic strategy is required. In this study, we investigated the antinociceptive effect of memantine and morphine on a vincristine-induced peripheral neuropathy model in rats.
Methods
Male Sprague-Dawley rats weighing 220-240 g were used in all experiments. Rats subsequently received daily intraperitoneal injections of either vincristine sulfate (0.1 ml/kg/day) or saline (0.1 ml/kg/day) over 12 days, immediately following behavioral testing. For assessment of mechanical allodynia, mechanical stimuli using von Frey filament was applied to the paw to measure withdrawal threshold. The effects of N-methyl-D-aspartate receptors antagonist (memantine; 2.5, 5, 10 mg/kg intraperitoneal), opioid agonist (morphine; 2.5, 5, 10 mg/kg intraperitoneal) and vehicle (saline) on vicristine-induced neuropathy were evaluated.
Results
Mechanical allodynia developed over the course of ten daily injections of vincristine relative to groups receiving saline at the same time. Morphine abolished the reduction in paw withdrawal threshold compared to vehicle and produced dose-responsiveness. Only the highest dose of memantine (10 mg/kg) was able to increase paw withdrawal threshold compared to vehicle.
Painful peripheral neuropathy is one of the main side effects induced by diverse classes of chemotherapeutic agents, including vincristine [1,2]. Vincristine is one of the most common chemotherapeutic drugs used to treat a wide variety of malignancies, including leukemia and lymphoma, and prevents tumor cell replication through alteration of cytoskeletal structure and disorientation of microtubules [3,4]. However, vincristine may also induce painful peripheral neuropathy. The chief clinical manifestation of vincristine-induced peripheral neuropathy is disturbance in both sensory and motor function [5,6]. Sensory disturbances range from mild tingling to spontaneous painful burning paresthesia and hypersensitivity to painful stimuli [7]. Vincristine-induced painful peripheral neuropathy is the major dose-limiting side effect and requires discontinuation of treatment, greatly impacting on the survival of cancer patients [8]. Moreover, the resulting symptoms, which frequently include moderate to severe pain, can often be disabling and cause significant loss of functional abilities and decreased quality of life [9].
Although it has been hypothesized that vincristine-induced neuropathic pain is due to neuronal toxicity and/or neurological disorder [10,11], the exact mechanism responsible is still unknown. Recently, Weng et al. [12] suggested a state of central sensitization develops in spinal wide dynamic range (WDR) neurons with repeated vincristine treatment that contributes to the neuropathic pain.
Unfortunately, neither prophylactic strategies nor symptomatic treatments of this chemotherapy-induced peripheral neuropathy (CIPN) have proven useful yet. Aspirin, ibuprofen and celebrex are commonly prescribed to patients to treat CIPN but show limited efficacy [13]. Furthermore, gabapentin, lamotrigine, nortriptyline and amitriptyline studies were disappointing in treating CIPN [14], and there have been no trials of opioids in patients with CIPN.
Data concerning the effectiveness of opioids on neuropathic pain have been controversial [15]. However, animal models [16] and controlled patient trials [17] suggest that µ-opioid receptor agonists are effective at attenuating neuropathic pain. Recently, opioid analgesics were recommended as second-line treatment that can be considered for first-line use in certain clinical circumstances [18].
Much evidence suggests that N-methyl-D-aspartate (NMDA) receptors play an important role in the generation of central sensitization, and in the development and maintenance of chronic pain [19,20]. On this basis, administration of NMDA receptor antagonists could be useful for the treatment of chronic pain [21,22]. Memantine, an amantadine derivative, is a noncompetitive NMDA antagonist that is better tolerated in patients because it is an open channel blocker with a fast off-rate compared with ketamine [23]. In the spinal nerve ligation model, memantine has been shown to be more effective in reversing allodynia and has produced the least motor impairment, as compared to MK-801 or ketamine [24]. The absence of confirmed treatments for CIPN makes the identification of effective alternative analgesics a necessity.
In this study, we investigated the antinociceptive effect of morphine and memantine on the vincristine-induced peripheral neuropathy model in rats.
All experiments followed the Guidelines on Ethical Standards for Investigation of Experimental Pain in Animals [25]. Bedding containing metabolized vincristine was treated as biohazardous waste and disposed of, according to the appropriate institutional guidelines.
Male Sprague-Dawley rats weighing 220-240 g were used in all experiments. Animals were acclimated to the laboratory environment for 5-7 days before being used in the study. While in the home cage environment, animals were allowed free access to a standard rat diet and tap water. Room temperature was maintained at 20-23℃ with 12:12 h light/dark cycle. Spontaneous behavior was observed in cages before starting experimental procedures, and rats showing aggressiveness or alterations in motility were discarded. An observer blinded to drug treatment conducted all behavioral assays.
The vincristine-induced peripheral neuropathy model induced by intraperitoneal (i.p.) injection was used in this experiment. Briefly, baseline responses to mechanical stimulation of the hindpaw were established on day zero (baseline). Rats subsequently received daily i.p. injections of either vincristine sulfate (0.1 ml/kg/day) or saline (0.1 ml/kg/day) over 12 days, immediately following behavioral testing. The treatment paradigm consisted of five daily injections, followed by a 2-day interval where no injections were administered, followed by five subsequent daily injections, as described previously [12]. In all studies, the investigator was blinded to drug treatments.
The following drugs were used in this study: vincristine sulfate (Tocris Cookson Ltd., Bristol, Avon, UK), memantine hydrochloride (Tocris), and morphine sulfate (Sigma Aldrich Co., St. Louis, MO, USA). All drugs were dissolved in 0.9% saline for systemic administration and administered in a volume of 0.1 ml/kg body weight. The dose of morphine and memantine were decided based on previous studies utilizing the neuropathic pain model [16,24].
Mechanical allodynia was assessed using von Frey filaments (Stoelting, Wood Dale, IL, USA), as described previously, to determine the 50% probability paw withdrawal threshold (PWT) [26]. Rats were placed in a separate transparent Plexi-glass chamber with a wire mesh floor underneath and acclimated to the test chambers for 30 min. The PWT was measured using von Frey filaments ranging from 0.4 to 15 g with a logarithmic increment. The filament was applied to the plantar hindpawn, and the force of each application was made optimal and consistent by having the filament bent to the same extent and maintained for 6 seconds. Withdrawal of paw or licking during the application was considered as a positive response. If the rat responded positively, the stiffer filament was used for the next trial; when no withdrawal or licking was observed, the less stiff one was used. The cut-off value was determined as 15 g, where the PWT for the rat was 15 g if the rat did not show any withdrawal or licking response to the application of a 15 g von Frey filament. Rats that demonstrated no allodynia (less than 4 g) were not excluded from this study.
On the day of experiments (on day 12), rats were allocated to receive one of the experimental drugs. The vehicle study was done using i.p. saline. Animals were tested only once, and all experiments were carried out by an observer blind to drug treatments.
The effects of NMDA receptor antagonist (memantine; 2.5, 5, 10 mg/kg i.p.) and opioid agonist (morphine; 2.5, 5, 10 mg/kg i.p.) were investigated in a vincristine-induced peripheral neuropathic pain state. The mechanical threshold measured before vincristine administration was regarded as the baseline threshold, while the withdrawal threshold measured immediately before i.p. delivery of drugs was regarded as control. The withdrawal threshold was determined at 15, 30, 45, 60, and 90 min after i.p. administration of experimental drugs.
Data were expressed as mean ± SEM. Time response data were presented as the withdrawal threshold in g. Dose-response data were presented as the percent of maximum possible effect (%MPE). Withdrawal threshold data from von Frey filament testing were converted to %MPE, according to the formula: %MPE = [(postdrug threshold - post-injured baseline threshold) / (cutoff threshold - post-injured baseline threshold)] × 100. In behavioral experiments, dose-response data were analyzed by one-way analysis of variance (ANOVA), with Scheffe for post hoc analysis. Differences in withdrawal threshold between multiple groups were analyzed by repeated measures ANOVA. P values of < 0.05 were considered statistically significant.
None of the vincristine-treated rats showed any motor dysfunction, guarding or obvious change in motor activity when observed in their home cages. All rats remained alert during the experiment. No difference between data recorded from the left or right hindpaws was found; therefore, withdrawal thresholds were presented as the mean of duplicate measurements, averaged across paws.
Administration of memantine (2.5, 5 and 10 mg/kg) at Day 12 also abolished the reduction in mechanical withdrawal thresholds only at the highest dose, returning PWT to control values (Fig. 2). The lower dose of memantine (2.5, 5 mg/kg) produced no significant reversal of tactile allodynia, while the highest dose of memantine (10 mg/kg) effectively increased mechanical withdrawal threshold compared to vehicle.
After repeated i.p. administration of vincristine, administration of morphine (2.5, 5 and 10 mg/kg) at Day 12 abolished the reduction in mechanical withdrawal thresholds in a dose dependent manner (Fig. 3). The effect of 2.5 mg/kg of morphine was not significantly different from control values recorded before morphine administration tactile threshold to mechanical stimulation. The higher doses of morphine (5 and 10 mg/kg) increased withdrawal threshold to tactile stimulation compared to vehicle.
In the present study, systemic administration of morphine produced significant anti-allodynic effects and dose-responsiveness. Lower doses of memantine (2.5, 5 mg/kg) produced no significant reversal of tactile allodynia, however the highest dose (10 mg/kg) effectively increased mechanical withdrawal threshold.
CIPN is a common and major dose limiting side effect of many chemotherapeutic agents. The most frequently reported agents include many older commonly used chemotherapeutic agents, such as platinum drugs, taxanes, epothilones and vinca alkaloids, but also newer agents such as bortezomib and lenolidamide [9,14]. The choice of chemotherapeutic agent, dosing schedule, type of cancer and presence of concomitant medical problems all affect the incidence and severity of chemotherapy-induced neuropathy [27,28]. The exact mechanism by which these agents cause CIPN is still unknown. Moreover, mechanisms are likely to be different and specific for each agent and, therefore, treatments may need to be agent-specific [14].
Experimental models of vincristine-induced peripheral neuropathic pain have been established in rodents using different systemic dosing schedules of vincristine. Present data were obtained using the vincristine model in rats [12]. In this model, it has been reported that in contrast to the change in response to mechanical stimulation, there was no significant change in latency of hind paw withdrawal responses to radiant heat stimuli after vincristine treatment [12,29].
In this study, we examined if two common analgesic drugs with clinical use would have an antinociceptive effect on neuropathic pain induced by vincristine. These analgesic drugs were selected because morphine is the reference opioid compound, while memantine is an NMDA antagonist used in several pain states.
NMDA receptors antagonist, memantine, has been used to treat Parkinson's disease, spasticity, convulsions, vascular dementia, and Alzheimer's disease with an extremely low incidence of side effects in human clinical trials [30,31]. It has also been reported that memantine is effective in the treatment of complex regional pain syndrome [32] and phantom limb pain [33]. In the present study, lower doses of memantine (2.5, 5 mg/kg) produced no significant reversal of tactile allodynia, however the higher dose of memantine (10 mg/kg) effectively increased mechanical withdrawal threshold. This result is in agreement with the effect of memantine in chronic pain models [34-36]. Medvedev et al. [36] reported that memantine caused a dose-dependent reduction in the intensity of formalin-induced grooming behavior and a 10 mg/kg dose attenuated tactile allodynia induced by sciatic nerve ligation. This result may reflect a contribution of the NMDA receptor system in vincristine-induced peripheral neuropathy. It is possible that under the present conditions higher doses of memantine would produce stronger effects. However, higher doses were not tested because of motor side effects (ataxia, incoordination and restlessness) that preclude efficient determination of tactile thresholds [36].
Recently, animal models and controlled patient trials suggest that µ-opioid receptor agonists are effective at attenuating neuropathic pain [16,17]. In this study, systemic morphine significantly increased the PWT compared to vehicle and produced dose-responsiveness. The effect of 2.5 mg/kg of morphine on tactile threshold to mechanical stimulation was not significantly different from control values. However, at doses of 5 and 10 mg/kg, morphine increased the withdrawal threshold to tactile stimulation when compared with values obtained before morphine treatment. Similar results have been published with other models of neuropathic pain [37,38]. Furthermore, a previous study demonstrated that morphine (8 mg/kg i.p.) suppressed vincristine-evoked mechanical allodynia relative to treatment with either vehicle or a lower dose (2.5 mg/kg i.p.) of morphine [29]. In contrast, another study [39] found opioid agonists, such as morphine (5 mg/kg, i.p.) administered alone on 5 consecutive days, did not modify hyperalgesia in vincristine rats. This discrepancy could be due to differences in study protocol and rat strain. The beneficial effects of morphine warrant further investigation of its mechanism of action in vincristine-induced neuropathic pain.
In conclusion, systemic memantine and morphine have an antiallodynic effect on the vincristine-induced peripheral neuropathy model in rats. Memantine and morphine may therefore offer an alternative approach to treatment of vincristine-induced neuropathic pain states. However, similar studies on various animal models are required to obtain a reliable oversight of the effect of theses drugs on vincristine-induced neuropathic pain in a clinical setting.
ACKNOWLEDGEMENTS
This study was supported by a grant (CRI10057-1) from the Chonnam National University Hospital Research Institute of Clinical Medicine.
References
1. Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002; 249:9–17. PMID: 11954874.

2. Postma TJ, Benard BA, Huijgens PC, Ossenkoppele GJ, Heimans JJ. Long-term effects of vincristine on the peripheral nervous system. J Neurooncol. 1993; 15:23–27. PMID: 8384253.
3. Himes RH, Kersey RN, Heller-Bettinger I, Samson FE. Action of the vinca alkaloids vincristine, vinblastine, and desacetyl vinblastine amide on microtubules in vitro. Cancer Res. 1976; 36:3798–3802. PMID: 954003.
4. Owellen RJ, Hartke CA, Dickerson RM, Hains FO. Inhibition of tubulin-microtubule polymerization by drugs of the Vinca alkaloid class. Cancer Res. 1976; 36:1499–1502. PMID: 1260766.
5. Casey EB, Jellife AM, Le Quesne PM, Millett YL. Vincristine neuropathy. Clinical and electrophysiological observations. Brain. 1973; 96:69–86. PMID: 4348690.
6. Weiden PL, Wright SE. Vincristine neurotoxicity. N Engl J Med. 1972; 286:1369–1370. PMID: 5027400.

7. Forman A. Peripheral neuropathy in cancer patients: clinical types, etiology, and presentation. Part 2. Oncology (Williston Park). 1990; 4:85–89. PMID: 2167114.
8. Sandler SG, Tobin W, Henderson ES. Vincristine-induced neuropathy. A clinical study of fifty leukemic patients. Neurology. 1969; 19:367–374. PMID: 5813374.

9. Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008; 44:1507–1515. PMID: 18571399.

10. Ogawa T, Mimura Y, Kato H, Ootsubo S, Murakoshi M. The usefulness of rabbits as an animal model for the neuropathological assessment of neurotoxicity following the administration of vincristine. Neurotoxicology. 2000; 21:501–511. PMID: 11022859.
11. Topp KS, Tanner KD, Levine JD. Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J Comp Neurol. 2000; 424:563–576. PMID: 10931481.

12. Weng HR, Cordella JV, Dougherty PM. Changes in sensory processing in the spinal dorsal horn accompany vincristine-induced hyperalgesia and allodynia. Pain. 2003; 103:131–138. PMID: 12749967.

13. Lynch JJ 3rd, Wade CL, Zhong CM, Mikusa JP, Honore P. Attenuation of mechanical allodynia by clinically utilized drugs in a rat chemotherapy-induced neuropathic pain model. Pain. 2004; 110:56–63. PMID: 15275752.

14. Kaley TJ, Deangelis LM. Therapy of chemotherapy-induced peripheral neuropathy. Br J Haematol. 2009; 145:3–14. PMID: 19170681.

15. Arnér S, Meyerson BA. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain. 1988; 33:11–23. PMID: 2454440.

16. Dellemijn P. Are opioids effective in relieving neuropathic pain? Pain. 1999; 80:453–462. PMID: 10342407.

17. Obara I, Makuch W, Spetea M, Schütz J, Schmidhammer H, Przewlocki R, et al. Local peripheral antinociceptive effects of 14-O-methyloxymorphone derivatives in inflammatory and neuropathic pain in the rat. Eur J Pharmacol. 2007; 558:60–67. PMID: 17204264.

18. Eisenberg E, McNicol ED, Carr DB. Efficacy and safety of opioid agonists in the treatment of neuropathic pain of nonmalignant origin: systematic review and meta-analysis of randomized controlled trials. JAMA. 2005; 293:3043–3052. PMID: 15972567.

19. Chizh BA, Headley PM. NMDA antagonists and neuropathic pain--multiple drug targets and multiple uses. Curr Pharm Des. 2005; 11:2977–2994. PMID: 16178757.

20. Eide PK. Wind-up and the NMDA receptor complex from a clinical perspective. Eur J Pain. 2000; 4:5–15. PMID: 10833550.

21. Buvanendran A, Kroin JS. Early use of memantine for neuropathic pain. Anesth Analg. 2008; 107:1093–1094. PMID: 18806007.

22. Finkel JC, Pestieau SR, Quezado ZM. Ketamine as an adjuvant for treatment of cancer pain in children and adolescents. J Pain. 2007; 8:515–521. PMID: 17434801.

23. Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004; 1:101–110. PMID: 15717010.

24. Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997; 280:829–838. PMID: 9023297.
25. Zimmermann M. Ethical guidelines for investigations on experimental pain in conscious animals. Pain. 1983; 16:109–110. PMID: 6877845.

26. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63. PMID: 7990513.

27. Cata JP, Weng HR, Lee BN, Reuben JM, Dougherty PM. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol. 2006; 72:151–169. PMID: 16493391.
28. Polomano RC, Bennett GJ. Chemotherapy-evoked painful peripheral neuropathy. Pain Med. 2001; 2:8–14. PMID: 15102312.

29. Rahn EJ, Makriyannis A, Hohmann AG. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br J Pharmacol. 2007; 152:765–777. PMID: 17572696.

30. Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ. Memantine Study Group. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003; 348:1333–1341. PMID: 12672860.

31. Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine Study Group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004; 291:317–324. PMID: 14734594.

32. Sinis N, Birbaumer N, Gustin S, Schwarz A, Bredanger S, Becker ST, et al. Memantine treatment of complex regional pain syndrome: a preliminary report of six cases. Clin J Pain. 2007; 23:237–243. PMID: 17314583.
33. Hackworth RJ, Tokarz KA, Fowler IM, Wallace SC, Stedje-Larsen ET. Profound pain reduction after induction of memantine treatment in two patients with severe phantom limb pain. Anesth Analg. 2008; 107:1377–1379. PMID: 18806054.

34. Carlton SM, Hargett GL. Treatment with the NMDA antagonist memantine attenuates nociceptive responses to mechanical stimulation in neuropathic rats. Neurosci Lett. 1995; 198:115–118. PMID: 8592634.

35. Suzuki R, Matthews EA, Dickenson AH. Comparison of the effects of MK-801, ketamine and memantine on responses of spinal dorsal horn neurones in a rat model of mononeuropathy. Pain. 2001; 91:101–109. PMID: 11240082.

36. Medvedev IO, Malyshkin AA, Belozertseva IV, Sukhotina IA, Sevostianova NY, Aliev K, et al. Effects of low-affinity NMDA receptor channel blockers in two rat models of chronic pain. Neuropharmacology. 2004; 47:175–183. PMID: 15223296.

37. Bulka A, Plesan A, Xu XJ, Wiesenfeld-Hallin Z. Reduced tolerance to the anti-hyperalgesic effect of methadone in comparison to morphine in a rat model of mononeuropathy. Pain. 2002; 95:103–109. PMID: 11790472.

38. Erichsen HK, Hao JX, Xu XJ, Blackburn-Munro G. Comparative actions of the opioid analgesics morphine, methadone and codeine in rat models of peripheral and central neuropathic pain. Pain. 2005; 116:347–358. PMID: 15982817.

39. Bujalska M, Makulska-Nowak H, Gumułka SW. Magnesium ions and opioid agonists in vincristine-induced neuropathy. Pharmacol Rep. 2009; 61:1096–1104. PMID: 20081245.

Fig. 1
Time course of hind paw withdrawal response to von Frey filaments after vincristine treatment. Data are presented as withdrawal threshold. Each line represents mean ± SEM of 8 rats. BL: baseline withdrawal threshold measured before vincristine treatment. Significant differences between saline (control) and vincristine treatment are indicated. *P < 0.05, †P < 0.01.
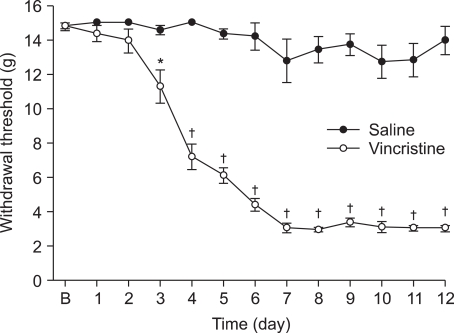
Fig. 2
Effects of intraperitoneal memantine for hindpaw withdrawal response to von Frey filaments after vincristine treatment. Data are presented as withdrawal threshold or percent of maximal possible effect (%MPE). Each line represents mean ± SEM of 6-7 rats. BL: baseline withdrawal threshold measured before vincristine treatment. Control data were measured immediately before intraperitoneal delivery of drug. Compared with vehicle (saline). *P < 0.01.
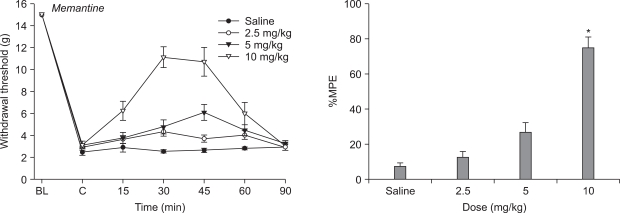
Fig. 3
Effects of intraperitoneal morphine for hindpaw withdrawal response to von Frey filaments after vincristine treatment. Data are presented as withdrawal threshold or percent of maximal possible effect (%MPE). Each line represents mean ± SEM of 6-7 rats. BL: baseline withdrawal threshold measured before vincristine treatment. Control data were measured immediately before intraperitoneal delivery of drug. Intraperitoneal morphine produced a dose-dependent increase in withdrawal threshold. Compared with vehicle (saline). *P < 0.05, †P < 0.01.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download