Abstract
Background
Neuropathic pain resulting from diverse causes is a chronic condition for which effective treatment is lacking. The goal of this study was to test whether dexamethasone exerts a preemptive analgesic effect with bupivacaine when injected perineurally in the spared nerve injury model.
Methods
Fifty rats were randomly divided into five groups. Group 1 (control) was ligated but received no drugs. Group 2 was perineurally infiltrated (tibial and common peroneal nerves) with 0.4% bupivacaine (0.2 ml) and dexamethasone (0.8 mg) 10 minutes before surgery. Group 3 was infiltrated with 0.4% bupivacaine (0.2 ml) and dexamethasone (0.8 mg) after surgery. Group 4 was infiltrated with normal saline (0.2 ml) and dexamethasone (0.8 mg) 10 minutes before surgery. Group 5 was infiltrated with only 0.4% bupivacaine (0.2 ml) before surgery. Rat paw withdrawal thresholds were measured using the von Frey hair test before surgery as a baseline measurement and on postoperative days 3, 6, 9, 12, 15, 18 and 21.
Intractable neuropathic pain often results from peripheral nerve injury or abnormal changes in the central nervous system caused by trauma, surgery, compression injuries, infection, metabolic diseases, ischemic or vascular diseases, immune diseases, or toxins [1,2]. Because such neuropathic pain is difficult to treat, prevention is considered the most effective treatment strategy. This process involves the administration of local anesthetics, opioids, and nonsteroidal anti-inflammatory drugs, glucocorticoids, alone or in combination, via local, epidural, intrathecal, or systemic routes [3,4]. Research has shown that this form of analgesia reduces pain and morbidity, minimizing analgesic requirements, and resulting in shorter hospitalizations [5-7]. However, some studies have failed to detect a difference between pre- and post-operative treatment groups with respect to preemptive analgesia [8-10]. Several studies have shown that perioperatively administered glucocorticoids have strong anti-inflammatory effects, reducing pain and swelling after oral surgery [11], orthopedic surgery, spinal surgery [12] and laparoscopic surgery [13]. Dexamethasone has long-acting (several days) effects that would appear to make it an ideal drug to combine with preemptive analgesia. Dexamethasone-containing bupivacaine microspheres may prolong the duration of nerve block in animal and human studies [14-16]. It has also been shown that adding methylprednisolone to local anesthetic may increase the duration of axillary brachial block [17]. In addition, Kelly et al. insisted that preemptive analgesia could be achieved using continuous or intermittent administration of single analgesics or analgesic combinations and treatment strategies that targeted the periphery, inputs along sensory axons, or central nervous system (CNS) sites [18].
Therefore, the aim of this study was to test whether the combination of dexamethasone and local anesthetics increases the preemptive analgesic effect of local anesthetics in the spared nerve injury model.
All animal experiments were performed in accordance with national legislation and followed the National Institutes of Health Guidelines regarding the care and use of animals for experimental procedures. The study protocol was approved by the Animal Care Committee of Asan Medical Center.
All procedures were performed in adult (180-200 g) male Sprague-Dawley rats, housed in an approved vivarium and maintained on a 12-hr light/12-hr dark cycle, with food and water provided ad libitum. The animals were received one week before surgery and habituated to the tester, the environment and the handling procedures prior to commencement of testing. Before surgery, mechanical nociceptive withdrawal responses were measured three times for adaptation to the testing environment. The mean value of three measurements was adopted as the pre-surgery value.
Neuropathic pain was induced following the method of the spared nerve injury rat model, as previously described [19].
Briefly, under enflurane (3.0 v%) anesthesia, an incision was made in the skin on the lateral surface of the thigh and the biceps femoris muscle was cut across, exposing the sciatic nerve and its three terminal branches: the sural, common peroneal and tibial nerves. The common peroneal and tibial nerves were tightly ligated with 5.0 silk and sectioned distal to the ligation, removing 2-4 mm of the distal nerve stump. Muscle and skin were enclosed in two layers, avoiding any contact with or stretching of the intact sural nerve.
Fifty animals were randomly assigned to five groups. Group 1 (control) was ligated but received no drugs. Group 2 was perineurally infiltrated (tibial and common peroneal nerves) with 0.4% bupivacaine (0.2 ml) and dexamethasone (0.8 mg) 10 minutes before surgery. Group 3 was perineurally infiltrated with 0.4% bupivacaine (0.2 ml) and dexamethasone (0.8 mg in same volume) 10 minutes after surgery. Group 4 was perineurally infiltrated with normal saline (0.2 ml) and dexamethasone (0.8 mg in same volume) 10 minutes before surgery. Group 5 was perineurally infiltrated with 0.4% bupivacaine (0.2 ml) without dexamethasone before surgery.
For perineural infiltration, the agent was placed directly onto the exposed nerve and allowed to flow onto the surrounding area, which was performed using finger pressure on a tuberculin syringe fitted with a 30-gauge needle (BD PrecisionGlide, Becton-Dickinson, Franklin Lakes, NJ).
Measurements of rat paw withdrawal threshold were collected before surgery as a baseline measurement and on postoperative days (POD) 3, 6, 9, 12, 15, 18 and 21. All behavioral experiments were conducted between 9:00 a.m. and 3:00 p.m. to reduce errors due to light/dark cycle variations. Each rat was placed individually in an inverted ventilated Plexiglas cage with a metal-mesh floor (8 × 8 mm) that provided access to the plantar surface of the hind paw, and allowed to habituate to its environment under non-restrained condition for 15 minutes.
According to the method described by Chaplan et al. [20], a series of eight calibrated von Frey filaments (0.41, 0.70, 1.20, 2.00, 3.63, 5.50, 8.50 and 15.10 g: Stoelting Company, Wood Dale, IL) was applied serially to the lateral plantar surface of the hind paw (sural nerve territory) in ascending order of strength, with sufficient force to cause gentle bending against the paw, and held for six seconds. A sharp withdrawal or paw flinching was considered a positive response. The mechanical stimulus producing a 50% likelihood of withdrawal was determined by using the up-down method. Mechanical allodynia was defined as a withdrawal threshold of less than 4 g.
Statistical analyses were performed with SigmaStat program (version 3.10; Systat software, Ill, USA). Multiple groups were compared using one-way analysis of variance (ANOVA) followed by pair-wise multiple comparisons using Tukey post hoc test when appropriate. In all comparisons, a P value of less than 0.05 was considered statistically significant. Data was presented as mean ± SE, unless otherwise stated.
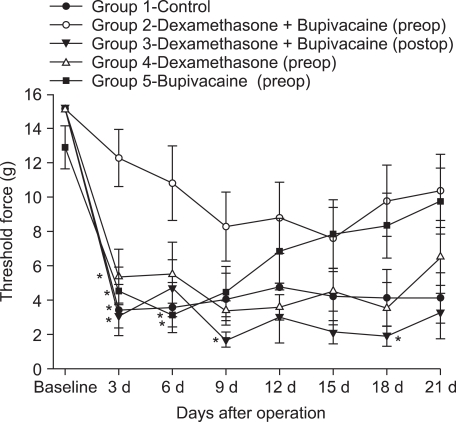
In groups 1, 3 and 4, mechanical force thresholds decreased from POD 3 to POD 21 (Fig. 1), whereas in group 5, mechanical force threshold decreased until POD 9, and then increased gradually thereafter until POD 21. In contrast, mechanical force threshold in group 2 did not decrease to less than the previously defined 4 g threshold during the 21-day postoperative period.
In a statistical comparison between groups, mechanical threshold for group 2 did not decrease significantly compared with those of groups 1, 3, 4 and 5 on POD 3, and with those of groups 1 and 4 on POD 6. On POD 9 and POD 18, group 2 mechanical threshold did not decrease significantly compared with that of group 3 (P < 0.05) (Fig. 1).
In this study, perineural infiltration with 0.8 mg dexamethasone and 0.4% bupivacaine (0.2 ml) prior to surgery prevented the development of mechanical allodynia through POD 21. In the group infiltrated with bupivacaine alone, mechanical allodynia developed early but gradually decreased after POD 9. Dexamethasone alone had no effect on the development of mechanical allodynia.
Neuropathic pain can develop through various mechanisms, such as a large increase in spontaneous (ectopic) discharges in dorsal root ganglia cell bodies and injured afferent fibers [21], cross excitation, damage to the high-threshold nociceptors (peripheral sensitization), collateral sprouting of intact fibers into denervated skin territories [22], amplification of the excitability of neurons within the CNS (central sensitization) [23], and central disinhibition due to the loss of an inhibitory mechanism [24].
Administration of local anesthetics into the skin before making an incision has been demonstrated to reduce post-operative pain in many kinds of elective surgery, such as inguinal herniorrhaphy [25], tonsillectomy [26], diagnostic laparoscopic procedures [27], gynecological procedure [28] and some orthopedic procedure [29]. In studies assessing the use of bupivacaine for reducing postoperative pain, Gordon et al. concluded that the observed blockade of sensory input extended beyond the local anesthetic duration of action [30]. They also suggested that the blockade of nociceptive input by a long-acting local anesthetic may decrease the development of central hyperexcitability, resulting in less pain and reducing the need for additional analgesics. In our study, we used 0.4% bupivacaine as the local anesthetic for preemptive analgesia because it has a longer half-life than lidocaine and offers potential advantages for the relief of postoperative pain [31].
Unlike in other studies [32-34], preemptive bupivacaine did not prevent a decrease in withdrawal thresholds early on, however it did increase thresholds after POD 9 in this study. Although the exact mechanism is unknown, possible reasons for this discrepancy may be differences in pain models and/or the method of drug administration. Judging from this result, infiltrated local anesthetics before nerve injury may not decrease postoperative pain, but may prevent the development of chronic neuropathic pain.
In addition to their anti-inflammatory action, steroids have other effects that contribute to their analgesic effect, including acting as reversible local anesthetics, stabilizing neural membranes, inhibiting neural peptide synthesis or action, suppressing ongoing or ectopic neural discharge and inhibiting sensitization of dorsal horn neurons [35]. Preoperative use of dexamethasone has been demonstrated to decrease postoperative pain in tonsillectomy, lumbar discectomy, laparoscopic cholecystectomy, and third molar teeth extraction [36-39]. According to another report [40], the addition of dexamethasone (8 mg) to a lidocaine (1.5%) solution for axillary brachial plexus block resulted in a significant increase in the duration of sensory and motor blocks, however the onsets of sensory and motor blockade were similar. Devor et al. reported corticosteroids may have a local anesthetic effect on the nerve [41]. Furthermore, Movafegh et al. suggested the additive effect of dexamethasone and lidocaine for axillary brachial plexus block may be related to this local anesthetic action [40].
Investigation of preemptive administration with bupivacaine or bupivacaine-methylprednisolone to the paravertebral muscles in patients undergoing lumbar discectomy revealed that pain decreased immediately after operation, and investigators asserted that injected corticosteroids acted against pain by inhibiting inflammation and preventing the secretion of neuropeptides that stimulate thin nerve fibers [41].
In addition to pain relief in postoperative patients, corticosteroids may suppress neuropathic pain. Large-dose oral glucocorticoids have been demonstrated to alleviate pain, hyperalgesia, and edema in complex regional pain syndrome patients [42]. Kozin et al. suggested that oral glucocorticoids exerted a prolonged effect even after discontinuation [43]. Furthermore, Kingery et al. [44] found that continuous infusion of methylprednisolone (3 mg/kg/d for 21 days), initiated after the development of neuropathic hyperalgesia, gradually reversed both heat and mechanical hyperalgesia over a period of several weeks, and an antihyperalgesic effect persisted for at least a week after discontinuing infusion. They also showed that continuous methylprednisolone infusion partially reversed nerve injury-evoked Fos protein expression in the dorsal horns, suggesting that glucocorticoids can inhibit spinal neuron hyperactivity induced by chronic sciatic nerve transaction. Topical injection of betamethasone has also been shown to inhibit the development of neuropathic pain in a rat model of spinal nerve transaction [45]. Betamethasone administration reduced the elevations of NF-κB, TNF-α, and IL-1β, and induced the expression of IL-10 in the brain, all of which were coincident with pain behavior in rats, suggesting that betamethasone may produce analgesic effects by modulating NF-κB and specific cytokines.
Consistent with these previous observations, the results of our study suggest that dexamethasone may inhibit neuropathic pain through a combination of its prolonged analgesic action and the increased effect of its local anesthetic action with that of bupivacaine. However, dexamethasone itself had no preemptive anti-neuropathic effect in our study. This may be due to the small sample sizes (group 2: n = 10, group 5: n = 10). It is also difficult to compare this result with those of other studies because of differences in pain models and method of drug administration. Clinically, the usual infiltration dosage is from 5-40 ml in 0.25-0.5% bupivacaine. Intravenous dosage of dexamethasone is 0.15-3 mg/kg and 8 mg of dexamethasone mixed with 1.5% lidocaine 34 ml was infiltrated via axillary brachial plexus blockade [42]. The dosage of dexamethasone (0.8 mg) used in this study wasn't small as a single bolus dose for an antineuropathic effect (e.g., through antihyperalgesic action). However, if the single bolus dosage is increased or continuous infusion is performed, dexamethasone could be effective in preventing the development of mechanical allodynia. Therefore, it is difficult to conclude that the administration of dexamethasone alone has no preemptive analgesic effect in the present study. Taken together, we suggest that the administration of dexamethasone in combination with bupivacaine increases the antiallodynic effect.
The analgesic effect of preoperative infiltration with both bupivacaine and dexamethasone was significantly better than with preoperative infiltration with bupivacaine alone for only the first 9 days after surgery. After 12 days post-surgery, there were no significant differences between preoperative infiltration with bupivacaine-dexamethasone and bupivacaine alone. In the present study, as in earlier studies, bupivacaine had an apparent preemptive analgesic effect when used alone, but was less effective than the combined analgesic action of bupivacaine and dexamethasone, especially early after injury. The mechanism of these results is unclear, but it can be postulated that it arises from the anti-inflammatory effect of dexamethasone.
In conclusion, preoperative infiltration of both dexamethasone and bupivacaine showed a significantly better analgesic effect than did infiltration of bupivacaine or dexamethasone alone in an SNI model, especially at the early stage after surgery. These results may indicate that dexamethasone acts synergistically or additively with bupivacaine to exert a preemptive analgesic effect, but has no preemptive analgesic effect on its own. Finally, preemptive analgesia with local anesthetics alone may be associated with a long-lasting decrease in mechanical allodynia.
References
1. Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999; 353:1959–1964. PMID: 10371588.

2. Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992; 355:75–78. PMID: 1370574.

3. Ersayli DT, Gurbet A, Bekar A, Uckunkaya N, Bilgin H. Effects of perioperatively administered bupivacaine and bupivacaine-methylprednisolone on pain after lumbar discectomy. Spine. 2006; 31:2221–2226. PMID: 16946657.

4. Mirzai H, Tekin I, Alincak H. Perioperative use of corticosteroid and bupivacaine combination in lumbar disc surgery: a randomized controlled trial. Spine. 2002; 27:343–346. PMID: 11840097.

5. Dahl JB, Kehlet H. The value of pre-emptive analgesia in the treatment of postoperative pain. Br J Anaesth. 1993; 70:434–439. PMID: 8499204.

7. Cervini P, Smith LC, Urbach DR. The effect of intraoperative bupivacaine administration on parenteral narcotic use after laparoscopic appendectomy. Surg Endosc. 2002; 16:1579–1582. PMID: 12045850.

8. Turner GA, Chalkiadis G. Comparison of preoperative with postoperative lignocaine infiltration on postoperative analgesic requirements. Br J Anaesth. 1994; 72:541–543. PMID: 8198905.

9. Dierking GW, Dahl JB, Kanstrup J, Dahl A, Kehlet H. Effect of pre- vs postoperative inguinal field block on postoperative pain after herniorrhaphy. Br J Anaesth. 1992; 68:344–348. PMID: 1642910.

10. Dahl JB, Hansen BL, Hjortsø NC, Erichsen CJ, Møiniche S, Kehlet H. Influence of timing on the effect of continuous extradural analgesia with bupivacaine and morphine after major abdominal surgery. Br J Anaesth. 1992; 69:4–8. PMID: 1637601.

11. Skjelbred P, Løkken P. Post-operative pain and inflammatory reaction reduced by injection of a corticosteroid. A controlled trial in bilateral oral surgery. Eur J Clin Pharmacol. 1982; 21:391–396. PMID: 7042372.

12. Holte K, Kehlet H. Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg. 2002; 195:694–712. PMID: 12437261.

13. Bisgaard T, Klarskov B, Kehlet H, Rosenberg J. Preoperative dexamethasone improves surgical outcome after laparoscopic cholecystectomy: a randomized double-blind placebo-controlled trial. Ann Surg. 2003; 238:651–660. PMID: 14578725.

14. Castillo J, Curley J, Hotz J, Uezono M, Tigner J, Chasin M, et al. Glucocorticoids prolong rat sciatic nerve blockade in vivo from bupivacaine microspheres. Anesthesiology. 1996; 85:1157–1166. PMID: 8916834.

15. Dräger C, Benziger D, Gao F, Berde CB. Prolonged intercostal nerve blockade in sheep using controlled-release of bupivacaine and dexamethasone from polymer microspheres. Anesthesiology. 1998; 89:969–979. PMID: 9778015.

16. Kopacz DJ, Lacouture PG, Wu D, Nandy P, Swanton R, Landau C. The dose response and effects of dexamethasone on bupivacaine microcapsules for intercostal blockade (T9 to T11) in healthy volunteers. Anesth Analg. 2003; 96:576–582. PMID: 12538215.

17. Stan T, Goodman EJ, Bravo-Fernandez C, Holbrook CR. Adding methylprednisolone to local anesthetic increases the duration of axillary block. Reg Anesth Pain Med. 2004; 29:380–381. PMID: 15305267.

18. Kelly DJ, Ahmad M, Brull SJ. Preemptive analgesia I: physiological pathways and pharmacological modalities. Can J Anaesth. 2001; 48:1000–1010. PMID: 11698320.

19. Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000; 87:149–158. PMID: 10924808.

20. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63. PMID: 7990513.

21. Devor M, Wall PD, Catalan N. Systemic lidocaine silences ectopic neuroma and DRG discharge without blocking nerve conduction. Pain. 1992; 48:261–268. PMID: 1589245.

22. Kingery WS, Vallin JA. The development of chronic mechanical hyperalgesia, autotomy and collateral sprouting following sciatic nerve section in rat. Pain. 1989; 38:321–332. PMID: 2812843.

23. Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983; 306:686–688. PMID: 6656869.

24. Woolf CJ, Wall PD. Chronic peripheral nerve section diminishes the primary afferent A-fibre mediated inhibition of rat dorsal horn neurones. Brain Res. 1982; 242:77–85. PMID: 7104735.

25. Tverskoy M, Cozacov C, Ayache M, Bradley EL Jr, Kissin I. Postoperative pain after inguinal herniorrhaphy with different types of anesthesia. Anesth Analg. 1990; 70:29–35. PMID: 2297102.

26. Jebeles JA, Reilly JS, Gutierrez JF, Bradley EL Jr, Kissin I. The effect of pre-incisional infiltration of tonsils with bupivacaine on the pain following tonsillectomy under general anesthesia. Pain. 1991; 47:305–308. PMID: 1784501.

27. Kato J, Ogawa S, Katz J, Nagai H, Kashiwazaki M, Saeki H, et al. Effects of presurgical local infiltration of bupivacaine in the surgical field on postsurgical wound pain in laparoscopic gynecologic examinations: a possible preemptive analgesic effect. Clin J Pain. 2000; 16:12–17. PMID: 10741813.

28. Hannibal K, Galatius H, Hansen A, Obel E, Ejlersen E. Preoperative wound infiltration with bupivacaine reduces early and late opioid requirement after hysterectomy. Anesth Analg. 1996; 83:376–381. PMID: 8694322.

29. McQuay HJ, Carroll D, Moore RA. Postoperative orthopaedic pain--the effect of opiate premedication and local anesthetic blocks. Pain. 1988; 33:291–295. PMID: 3419836.

30. Gordon SM, Dionne RA, Brahim J, Jabir F, Dubner R. Blockade of peripheral neuronal barrage reduces postoperative pain. Pain. 1997; 70:209–215. PMID: 9150295.

31. Roberge CW, McEwen M. The effects of local anesthetics on postoperative pain. AORN J. 1998; 68:1003–1012. PMID: 9864591.

32. Kissin I, Lee SS. Effects of long-term nerve blockade in the spared nerve injury model. Anesthesiology. 2004; 101:806–807. PMID: 15329619.

33. Ririe DG, Barclay D, Prout H, Tong C, Tobin JR, Eisenach JC. Preoperative sciatic nerve block decreases mechanical allodynia more in young rats: is preemptive analgesia developmentally modulated? Anesth Analg. 2004; 99:140–145. PMID: 15281520.

34. Suter MR, Papaloïzos M, Berde CB, Woolf CJ, Gilliard N, Spahn DR, et al. Development of neuropathic pain in the rat spared nerve injury model is not prevented by a peripheral nerve block. Anesthesiology. 2003; 99:1402–1408. PMID: 14639156.

35. Manchikanti L. Role of neuraxial steroids in interventional pain management. Pain Physician. 2002; 5:182–199. PMID: 16902669.

36. Baxendale BR, Vater M, Lavery KM. Dexamethasone reduces pain and swelling following extraction of third molar teeth. Anaesthesia. 1993; 48:961–964. PMID: 8250191.

37. Giannoni C, White S, Enneking FK. Does dexamethasone with preemptive analgesia improve pediatric tonsillectomy pain? Otolaryngol Head Neck Surg. 2002; 126:307–315. PMID: 11956540.

38. Lee C, Kim TY. The effect of preoperative dexamethasone administration, according to age and gender on postoperative pain in patients who undergo laparoscopic cholecystectomy. Korean J Pain. 2008; 21:51–56.

39. Tom LW, Templeton JJ, Thompson ME, Marsh RR. Dexamethasone in adenotonsillectomy. Int J Pediatr Otorhinolaryngol. 1996; 37:115–120. PMID: 8894809.

40. Movafegh A, Razazian M, Hajimaohamadi F, Meysamie A. Dexamethasone added to lidocaine prolongs axillary brachial plexus blockade. Anesth Analg. 2006; 102:263–267. PMID: 16368840.

41. Devor M, Govrin-Lippmann R, Raber P. Corticosteroids suppress ectopic neural discharge originating in experimental neuromas. Pain. 1985; 22:127–137. PMID: 4047699.

42. Christensen K, Jensen EM, Noer I. The reflex dystrophy syndrome response to treatment with systemic corticosteroids. Acta Chir Scand. 1982; 148:653–655. PMID: 6763435.
43. Kozin F, McCarty DJ, Sims J, Genant H. The reflex sympathetic dystrophy syndrome. I. Clinical and histologic studies: evidence for bilaterality, response to corticosteroids and articular involvement. Am J Med. 1976; 60:321–331. PMID: 56891.
44. Kingery WS, Agashe GS, Sawamura S, Davies MF, Clark JD, Maze M. Glucocorticoid inhibition of neuropathic hyperalgesia and spinal Fos expression. Anesth Analg. 2001; 92:476–482. PMID: 11159254.

45. Xie W, Luo S, Xuan H, Chou C, Song G, Lv R, et al. Betamethasone affects cerebral expressions of NF-kappaB and cytokines that correlate with pain behavior in a rat model of neuropathy. Ann Clin Lab Sci. 2006; 36:39–46. PMID: 16501235.
Fig. 1
Mechanical thresholds during the first 21 days postoperation. Fifty animals were randomly assigned to five groups. Rats were perineurally infiltrated (tibial and common peroneal nerves) with either saline, or 0.4% bupivacaine (0.2 ml) and/or dexamethasone (0.8 mg) 10 minutes before surgery. Another group of rats were perineurally infiltrated with 0.4% bupivacaine (0.2 ml) and dexamethasone (0.8 mg) after surgery. Measurements of rat paw withdrawal threshold were collected before surgery as a baseline measurement and on postoperative days 3, 6, 9, 12, 15, 18 and 21. Data are expressed as mean ± SEM. n = 10. *P < 0.05 compared with group 2.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download