Abstract
The evolution of brain imaging techniques over the last decade has been remarkable. Along with such technical developments, research into chronic pain has made many advances. Given that brain imaging is a non-invasive technique with great spatial resolution, it has played an important role in finding the areas of the brain related to pain perception as well as those related to many chronic pain disorders. Therefore, in the near future, brain imaging techniques are expected to be the key to the discovery of many unknown etiologies of chronic pain disorders and to the subjective diagnoses of such disorders.
The ancient Greek philosopher Epictetus stated in his book, The Enchiridion, that "For death or pain is not formidable, but the fear of pain or death." [1]. Setting the philosophical interpretation of the statement aside and focusing on Epictetus' insight that the fear of 'pain' is greater than the fear of 'death', it becomes clear that throughout the ages pain has been one of the most distressing miseries that a human being may experience. There are two types of pain: acute pain and chronic pain. Chronic pain is that which brings agony to mankind from childhood to old age. According to a recent survey, 20% of the European population suffers from chronic pain [2]. In Korea, more than 10% of the adult population suffers from chronic pain disorder. It has become commonly accepted that the incidence may be substantially underestimated. In 2006, the second largest-selling pharmaceutical product category in Korea was analgesics [3]. Nearly 65% of people with chronic pain reported that pain interferes with their daily life [4]. In addition to the impairment of life functions, chronic pain has a high comorbidity rate. The majority of chronic pain sufferers have a greater incidence of other disorders, including mood disorders, anxiety disorders, and sleep disorders [5-10].
However, while chronic pain afflicts a considerable number of people, there is insufficient understanding and insight into its unique characteristics [11]. Moreover, although chronic pain is studied as a component of a number of research areas, such as anesthesiology and pain medicine, anatomy, neurology, and psychiatry, thus far there has been no solid and objective indicator established for the diagnosis of pain. The fact that pain is a very subjective experience is one of the reasons for this. Brain imaging may serve as a viable solution to the subjectivity problem of pain, as brain imaging is non-invasive and facilitates both a structural and functional approach to understanding chronic pain [12]. Over the past ten years, numerous studies of chronic pain have been carried out using brain imaging techniques. Through those studies, pain researchers were able to establish a pain matrix, which is comprised of the brain areas related to pain perception. These areas include the primary somatosensory cortex (S1), secondary somatosensory cortex (S2), insula, anterior cingulate cortex (ACC), primary motor cortex, supplementary motor area (SMA), thalamus, basal ganglia, midbrain, cerebellum, prefrontal cortex (PFC), and the posterior parietal cortex [13-16]. Therefore, reviewing chronic pain studies based on brain imaging methods will hasten the standardization of the diagnosis of chronic pain and will provide directions for further study.
The imaging method known as fMRI is one of the most commonly used imaging methods in chronic pain research. There are many chronic pain disorders of which the etiology is unknown due to a lack of understanding of the structural abnormalities and their association with different disorders. fMRI is a very useful non-invasive technique for obtaining information related to neural networks and their activities [17,18].
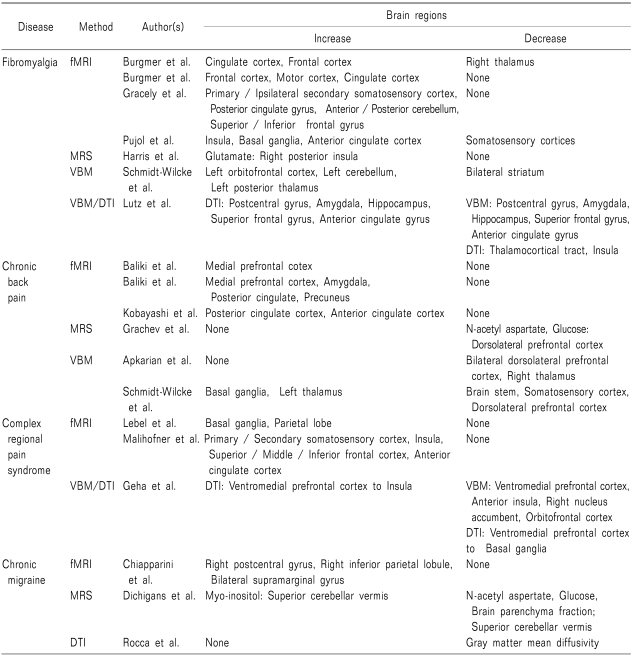
Fibromyalgia is a common chronic pain disorder. It is characterized by allodynia all over the body and a feeling of exhaustion with no specific cause. Fibromyalgia commonly accompanies other disorders, such as mood disorder or sleep disorder. Fibromyalgia studies are mostly focused on abnormal activities that occur in the central nervous system. Numerous studies have reported that fibromyalgia patients have distinctively more activity in the pain matrix compared to control groups. According to the latest research, predictions of pain and perceptions of pain both have a meaningful positive correlation with hyperactivity in the brain areas of the motor cortex and cingulate cortex; the patient group in this line of research showed distinct activity in the frontal and temporal areas [19]. Moreover, increased activity was noted in the medial frontal cortex, cerebellum, dorsal ACC, dorsolateral PFC, and claustrum, all of which are areas related to pain anticipation and attention and all of which have a significant correlation with pain catastrophe [20]. The areas of the anterior and mid-cingulate cortex, middle frontal cortex, SMA, thalamus, anterior insula, and basal ganglia displayed more activity in fibromyalgia patients [21,22]. Overall, fibromyalgia patients showed increased neural activity in pain-related areas.
Chronic back pain is a widespread pain disorder that afflicts a broad range of the population. It has been estimated that nearly 80% the human population has experienced chronic back pain at least once in their life [23]. However, the pathological causes of this type of pain remain unidentified. In this area of research, neuro-imaging techniques have been used to investigate the causes. According to Baliki et al. [24], chronic back pain influences the deactivation of the default mode network (DMN) during the resting state. It has also been reported that the posterior cingulate cortex (PCC) plays an important role as an affective component of pain mediation, as much as the ACC, which is known to be responsible for pain mediation [25]. In another study, the medial PFC showed significantly increased activity in chronic back pain patients [26].
Complex regional pain syndrome (CRPS) is a chronic pain disorder that accompanies sensory, motor, and autonomic dysfunctions. Its origin as well has yet to be identified. This lack of knowledge delays the proper type of treatment for CRPS patients [26-28]. Maihöfner et al. [26] observed activations in the S1 and S2 cortex, insula, PFC, and in the ACC during pin-prick stimulation of CRPS patients who experience hyperalgesia. They found that the S2 cortex is also involved in the processing of non-painful stimuli. Another report found that children and teenagers with CRPS showed distinct activation patterns in the central nervous system when stimulated with mechanical and thermal stimuli; especially noted was decreased activity during a painful state [29].
There are many other chronic pain studies involving patients with unidentified chronic pain. Malinen et al. [30] studied 10 patients who suffered severely from unknown chronic pain. They found that during a resting period, these patients showed abnormal temporal and spatial fluctuations and connectivity disturbances in the pain matrix, particularly the lower insula and the ACC. Diabetic patients who suffered from neuropathic pain also displayed significantly different brain activity from healthy control subjects during the resting period [31]. Disturbances in the DMN of chronic pain patients have been noted in many studies [24,30,31]. Another report showed that when patients feel depressed, the activities of the PFC, subgenual ACC, and hippocampus are all more intense, as is the level of perceived pain [32]. Such studies give deeper insight into the high correlation between chronic pain and mood disorders.
MRS allows us to explore not only the structural information but also the biochemical information of the brain. There are few MRS studies pertaining to chronic pain. A handful of studies suggest the important role of glutamate in chronic pain. Harris et al. [33] reported that the levels of glutamate and combined glutamate, both known to play an important role in pain neurotransmission, were significantly higher in the right posterior insula of fibromyalgia patients. They also found that glutamate and combined glutamate levels are negatively correlated with pain threshold. Moreover, glutamergic abnormalities were found in the ACC and insula of migraine patients. Such abnormalities were suggested to be responsible for the chronic nature of the patients' migraines [34]. There have also been reports that N-acetyl aspartate and glucose were decreased in the dorsolateral PFC of chronic back pain patients [35,36]. When such findings achieve more reliable congruence, accounting for this information may lead to more objective diagnoses of chronic patients.
Using VBM, the volume of brain tissue can be measured. By comparing the gray matter volume of chronic pain patients to that of healthy controls, structural differences, if they exist, in the brains of pain patients can be estimated. Research on chronic pain using VBM includes a few conflicting results, but thus far, researchers generally agree that chronic pain patients have decreased gray matter volume in the regions considered as the pain matrix. Chronic tension-type headache patients showed an overall decrease in their gray matter volume in the pain matrix [37], while chronic back pain patients also showed reduced gray matter density in the bilateral dorsolateral PFC and right thalamus compared to healthy controls [38]. CRPS patients also showed overall decrease in gray matter volume. Gray matter reduction in the right insula, right ventromedial PFC, and right nucleus accumbens was particularly more severe compared to that in other regions [39]. Gray matter intensity in fibromyalgia patients was significantly reduced in the postcentral gyrus, amygdala, hippocampus, superior frontal gyrus, and anterior cingulate gyrus as well [40]. Burgmer et al. [41] also reported decreased gray matter volume in the PFC, amygdala, and ACC. A study of female fibromyalgia patients also found significantly decreased gray matter density in the left parahippocampal gyrus, bilateral mid/posterior cingulate gyrus, left insula, and medial frontal cortex [42]. Kim et al. [43] also reported decreased gray matter volumes in the bilateral insula, bilateral motor/premotor cortex, bilateral PFC, left dorsal ACC, right dorsal posterior cingulate cortex, right inferior and superior parietal cortex, and orbitofrontal cortex in migraine patients. Amputees who experience phantom limb sensations had reduced gray matter in the anterior and posterior cingulate regions, SMA, and midbrain [44]. However, other studies report increased gray matter volume in some brain regions, as well as reports of gray matter reduction. Chronic back pain patients showed substantial decreases in the somatosensory cortex and brainstem, whereas the volume of gray matter in the bilateral basal ganglia and left thalamus increased significantly [45]. Another report found that fibromyalgia patients had increased gray matter volumes in their left orbitofrontal cortex, left cerebellum and bilateral striatum with decreased gray matter volume in their right superior temporal gyrus and left posterior thalamus [46]. In addition, Teutsch et al. [47] observed a number of modulations, including an increase in gray matter in the middle cingulate cortex and somatosensory cortex when noxious stimuli were applied repetitively to healthy subjects. Although it is too soon to conclude anything regarding a tendency toward an increase in gray matter in chronic pain patients at this point, increased gray matter volumes were observed mostly in the left hemisphere.
The DTI method provides quantitative information on chronic pain processing in the central nervous system by monitoring the motions of water molecules. It can be an effective marker for the structural evolution of long-term disease [48]. Chronic pain studies using a DTI technique are not yet as prevalent as studies that utilize other imaging techniques. Using a DTI method, Rocca et al. [49] investigated normal-appearing brain tissue in migraine patients. They reported no significant differences in fractional anisotropy (FA) and mean diffusivity. Later in 2006, they investigated the influence of normal-appearing white matter and gray matter separately in migraine patients. They found a reduction in the mean diffusivity of the gray matter in migraine patients, whereas no significant difference was noted in the mean diffusivity of their white matter. Moreover, no distinct FA difference was noted in both the white and gray matter of these patients [50]. Lutz et al. [40] reported that white matter FA was decreased in the bilateral thalamus, bilateral insula and thalamocortical tracts, whereas it was increased in the postcentral gyrus, amygdala, hippocampus, superior frontal gyrus and anterior cingulate gyrus. They also suggested that the perception of both pain intensity and fatigue was associated with the white matter FA in certain regions of the pain matrix, implying its possible application in pain diagnosis. CRPS patients showed decreased white matter FA in left callosal fiber tract in lateral ACC [39]. If structural changes are consistently observed in patients, diagnosing chronic pain disorders and estimating the degree of suffering can be standardized.
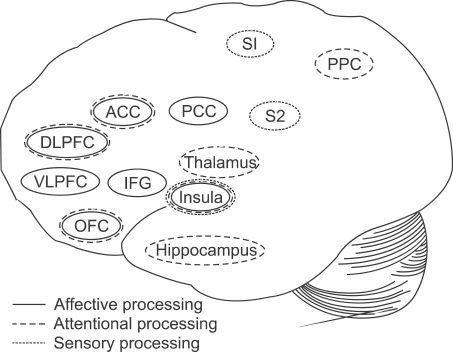
Pain-related brain areas have different patterns of activity during the processing of pain with distinct characteristics. Related to the affective aspects of pain processing, areas such as the insula, inferior frontal gyrus, orbitofrontal cortex, ventrolateral and dorsolateral prefrontal cortex, PCC, and ACC show discriminative activity [18,20,32,51,52]. The S1 and S2 areas as well as the insula are reportedly essential in the sensory processing of pain, and many feel that S2 is also important for affective processing [20,52-55]. Pain processing related to attention has been associated with significantly altered activity in the thalamus, insula, hippocampus, ACC, orbitofrontal cortex, dorsolateral prefrontal cortex, and posterior parietal cortex (Fig. 1) [20,53,55,56]. In addition, it is important to note that among these areas, the insula has been continually reported to be significantly active during all of the processes of pain. These distinctions in pain processing may be essential for treating chronic pain patients with distinct symptoms. Moreover, as these processes cover various functions of the central nervous system, multidisciplinary approaches will be critical.
For the past decade, brain imaging techniques have made remarkable progress [16] and have contributed to advances in chronic pain research. Overall, the fMRI studies report increased activities in pain patients' pain matrix, regardless of disorders. There are some studies that reports increased volume of gray matter of chronic pain group, but most reports have agreed that gray matter volume in the chronic pain patient is decreased. Most frequently observed pain-related brain regions that showed abnormal brain function or structure were insula, thalamus, cingulate cortex, basal ganglia and frontal cortex (Table 1).
With these imaging methods, researchers have been able to understand the functional and structural mechanisms of chronic pain. However, a number of fundamental questions remain unanswered, and multitudes still suffer from chronic pain without clear etiological information. Although pain researchers are now much more conversant in their comprehension of chronic pain compared to where they were years ago, more insight is required to unravel the nature of chronic pain and to apply the findings so as to treat or diagnose those who suffer from chronic pain. Therefore, in chronic pain research, active multidisciplinary approaches are necessary to get to the core of the mechanisms of chronic pain.
References
1. Epictetus . Discourses of epictetus. 2006. Sioux Falls: NuVision Publications, LLC;p. 98.
2. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006; 10:287–333. PMID: 16095934.

3. Kim B. KHIDI statistics brief. 2008. vol. 1. Seoul: Korea Health Industry Development Institute;p. 5.
4. Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, et al. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiol Scand. 2008; 52:132–136. PMID: 17976220.

5. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry. 2003; 54:399–409. PMID: 12893114.

6. Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004; 5(Suppl 1):S9–S27. PMID: 14996227.

7. Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005; 6:533–544. PMID: 15995724.

8. Broggi G. Pain and psycho-affective disorders. Neurosurgery. 2008; 62(6 Suppl 3):901–919. PMID: 18695578.

9. Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and neuropathic pain. Front Biosci. 2009; 14:5291–5338. PMID: 19482616.

10. Turk DC, Audette J, Levy RM, Mackey SC, Stanos S. Assessment and treatment of psychosocial comorbidities in patients with neuropathic pain. Mayo Clin Proc. 2010; 85(3 Suppl):S42–S50. PMID: 20194148.

11. Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009; 87:81–97. PMID: 18952143.

12. Tracey I. Neuroimaging of pain mechanisms. Curr Opin Support Palliat Care. 2007; 1:109–116. PMID: 18685351.

13. Ochsner KN, Zaki J, Hanelin J, Ludlow DH, Knierim K, Ramachandran T, et al. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Soc Cogn Affect Neurosci. 2008; 3:144–160. PMID: 19015105.

15. May A. Neuroimaging: visualising the brain in pain. Neurol Sci. 2007; 28(Suppl 2):S101–S107. PMID: 17508154.

16. Seifert F, Maihöfner C. Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cell Mol Life Sci. 2009; 66:375–390. PMID: 18791842.

17. Chiapparini L, Grazzi L, Ferraro S, Mandelli ML, Usai S, Andrasik F, et al. Functional-MRI evaluation of pain processing in chronic migraine with medication overuse. Neurol Sci. 2009; 30(Suppl 1):S71–S74. PMID: 19415430.

18. Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006; 26:12165–12173. PMID: 17122041.

19. Burgmer M, Pogatzki-Zahn E, Gaubitz M, Stüber C, Wessoleck E, Heuft G, et al. Fibromyalgia unique temporal brain activation during experimental pain: a controlled fMRI Study. J Neural Transm. 2010; 117:123–131. PMID: 19937376.

20. Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004; 127:835–843. PMID: 14960499.

21. Burgmer M, Gaubitz M, Konrad C, Wrenger M, Hilgart S, Heuft G, et al. Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosom Med. 2009; 71:566–573. PMID: 19414621.

22. Pujol J, López-Solà M, Ortiz H, Vilanova JC, Harrison BJ, Yücel M, et al. Mapping brain response to pain in fibromyalgia patients using temporal analysis of FMRI. PLoS One. 2009; 4:e5224. PMID: 19381292.

23. Andersson GB. Epidemiology of low back pain. Acta Orthop Scand Suppl. 1998; 281:28–31. PMID: 9771538.

24. Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008; 28:1398–1403. PMID: 18256259.

25. Kobayashi Y, Kurata J, Sekiguchi M, Kokubun M, Akaishizawa T, Chiba Y, et al. Augmented cerebral activation by lumbar mechanical stimulus in chronic low back pain patients: an FMRI study. Spine. 2009; 34:2431–2436. PMID: 19789470.

26. Maihöfner C, Forster C, Birklein F, Neundörfer B, Handwerker HO. Brain processing during mechanical hyperalgesia in complex regional pain syndrome: a functional MRI study. Pain. 2005; 114:93–103. PMID: 15733635.

27. Ok SJ, Yang JY, Son JH, Jeong WJ, Lee YS, Kim WY, et al. Management of complex regional pain syndrome type 1 with total spinal block. Korean J Pain. 2010; 23:70–73. PMID: 20552078.

28. Choi YS, Lee MG, Lee HM, Lee CJ, Jo JY, Jeon SY, et al. Epidemiology of complex regional pain syndrome: a retrospective chart review of 150 Korean patients. J Korean Med Sci. 2008; 23:772–775. PMID: 18955780.

29. Lebel A, Becerra L, Wallin D, Moulton EA, Morris S, Pendse G, et al. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain. 2008; 131:1854–1879. PMID: 18567621.

30. Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, et al. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci USA. 2010; 107:6493–6497. PMID: 20308545.

31. Cauda F, Sacco K, Duca S, Cocito D, D'Agata F, Geminiani GC, et al. Altered resting state in diabetic neuropathic pain. PLoS One. 2009; 4:e4542. PMID: 19229326.

32. Berna C, Leknes S, Holmes EA, Edwards RR, Goodwin GM, Tracey I. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry. 2010; 67:1083–1090. PMID: 20303069.

33. Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009; 60:3146–3152. PMID: 19790053.

34. Prescot A, Becerra L, Pendse G, Tully S, Jensen E, Hargreaves R, et al. Excitatory neurotransmitters in brain regions in interictal migraine patients. Mol Pain. 2009; 5:34. PMID: 19566960.

35. Grachev ID, Fredrickson BE, Apkarian AV. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain. 2000; 89:7–18. PMID: 11113288.

36. Grachev ID, Fredrickson BE, Apkarian AV. Brain chemistry reflects dual states of pain and anxiety in chronic low back pain. J Neural Transm. 2002; 109:1309–1334. PMID: 12373563.

37. Schmidt-Wilcke T, Leinisch E, Straube A, Kämpfe N, Draganski B, Diener HC, et al. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005; 65:1483–1486. PMID: 16275843.

38. Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004; 24:10410–10415. PMID: 15548656.

39. Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008; 60:570–581. PMID: 19038215.

40. Lutz J, Jäger L, de Quervain D, Krauseneck T, Padberg F, Wichnalek M, et al. White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis Rheum. 2008; 58:3960–3969. PMID: 19035484.

41. Burgmer M, Pogatzki-Zahn E, Gaubitz M, Wessoleck E, Heuft G, Pfleiderer B. Altered brain activity during pain processing in fibromyalgia. Neuroimage. 2009; 44:502–508. PMID: 18848998.

42. Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007; 27:4004–4007. PMID: 17428976.

43. Kim JH, Suh SI, Seol HY, Oh K, Seo WK, Yu SW, et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008; 28:598–604. PMID: 18422725.

44. Draganski B, Moser T, Lummel N, Gänssbauer S, Bogdahn U, Haas F, et al. Decrease of thalamic gray matter following limb amputation. Neuroimage. 2006; 31:951–957. PMID: 16520065.

45. Schmidt-Wilcke T, Leinisch E, Gänssbauer S, Draganski B, Bogdahn U, Altmeppen J, et al. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006; 125:89–97. PMID: 16750298.

46. Schmidt-Wilcke T, Luerding R, Weigand T, Jürgens T, Schuierer G, Leinisch E, et al. Striatal grey matter increase in patients suffering from fibromyalgia--a voxel-based morphometry study. Pain. 2007; 132(Suppl 1):S109–S116. PMID: 17587497.
47. Teutsch S, Herken W, Bingel U, Schoell E, May A. Changes in brain gray matter due to repetitive painful stimulation. Neuroimage. 2008; 42:845–849. PMID: 18582579.

48. Rovaris M, Agosta F, Pagani E, Filippi M. Diffusion tensor MR imaging. Neuroimaging Clin N Am. 2009; 19:37–43. PMID: 19064198.

49. Rocca MA, Colombo B, Inglese M, Codella M, Comi G, Filippi M. A diffusion tensor magnetic resonance imaging study of brain tissue from patients with migraine. J Neurol Neurosurg Psychiatry. 2003; 74:501–503. PMID: 12640073.

50. Rocca MA, Ceccarelli A, Falini A, Tortorella P, Colombo B, Pagani E, et al. Diffusion tensor magnetic resonance imaging at 3.0 tesla shows subtle cerebral grey matter abnormalities in patients with migraine. J Neurol Neurosurg Psychiatry. 2006; 77:686–689. PMID: 16614037.

51. Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997; 277:968–971. PMID: 9252330.

52. Sengupta S, Kumar D. Pain and emotion: relationship revisited. German J Psychiatry. 2005; 8:85–93.
53. Peyron R, Garcia-Larrea L, Grégoire MC, Costes N, Convers P, Lavenne F, et al. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain. 1999; 122:1765–1780. PMID: 10468515.
54. Flor H, Braun C, Elbert T, Birbaumer N. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett. 1997; 224:5–8. PMID: 9132689.

55. López-Solà M, Pujol J, Hernández-Ribas R, Harrison BJ, Ortiz H, Soriano-Mas C, et al. Dynamic assessment of the right lateral frontal cortex response to painful stimulation. Neuroimage. 2010; 50:1177–1187. PMID: 20080188.

56. Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002; 125:310–319. PMID: 11844731.

Fig. 1
This illustration shows the areas in the brain that process some discriminative components of pain perception, including affective processing, attentional processing, and sensory processing. The thalamus, insula, hippocampus, orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), and posterior parietal cortex (PPC) are distinctly active during the attentional processing of pain perception. During sensory processing, the primary and secondary somatosensory cortices (S1, S2) and the insula are involved. The activity of the insula, inferior frontal gyrus (IFG), OFC, ventrolateral prefrontal cortex (VLPFC), DLPFC, posterior cingulate cortex (PCC) and ACC were distinct during the affective processing of pain. Many researchers hold that the S2 area is also involved in this process.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download