Abstract
Background
To develop a rabbit epidural steroid injection (ESI) model for analyzing steroid retention in the tissue, and to assess the difference in steroid retention in the model according to the location and time elapsed after ESI.
Methods
Fluoroscopy-guided ESI was performed using the interlaminar approach between the lowest two lumbar segments in 13 female New Zealand white rabbits. Four rabbits were allocated to each of three different groups according to the time of sacrifice: 3, 7, and 15 days post-ESI; the remaining rabbit was sacrificed immediately post-ESI to obtain baseline data. After sacrifice, two segments were harvested: the lowest two lumbar vertebrae and another two lumbar vertebrae immediately above these. The residual steroid amount (RSA) and residual steroid concentration (RSC) in the collected spinal columns were analyzed. A linear mixed model was used to compare RSAs and RSCs between the injected and adjacent segments, and among the number of days until sacrifice; P < 0.05 was considered statistically significant.
Results
Both RSA and RSC of the injected segment were significantly higher than those of the adjacent segment (P < 0.001, both). The RSA and RSC significantly decreased over time (P = 0.009 and P = 0.016, respectively).
Conclusions
The developed rabbit ESI model verified that significantly more steroid was retained at the injected segment than at the adjacent segment and the residual steroid decreased over time. This model could be useful not only for comparing current steroid medications, but also for developing new, better steroid formulations.
Epidural steroid injection (ESI) is an important treatment for patients with radicular lower back or neck pain. Due to the increased life expectancy and high prevalence of chronic back pain in older people [1], nonsurgical interventional procedures for spinal pain have significantly increased. In the United States, ESI usage increased 130% from 2000 to 2011 in Medicare beneficiaries; ESI was performed for 4,879 per 100,000 patients in 2011 [2].
Various chronic back pain conditions, which include herniated disc disease, spinal stenosis, and post-lumbar surgery syndrome, are conventional candidates for ESI. Multiple studies have shown robust evidence of the short-term (< 6 mo) effectiveness of ESI in patients with disc herniation [3,4]. For treatment, drugs are directly delivered into the epidural space under fluoroscopic or computed tomographic guidance. Steroids are generally used for their anti-inflammatory effects, but exert variable adverse systemic effects, including suppression of the hypothalamus-pituitary-adrenal axis, Cushing syndrome, osteoporosis [5], fluid retention, and hyperglycemia. Therefore, current guidelines suggest limiting the frequency of interventional procedures. The American Society of Interventional Pain Physicians recommends that the interval between epidural injections be 2 months or longer, assuming that more than 50% relief is obtained for 2 months. However, a previous randomized, double-blind trial [6] showed widespread use of more frequent epidural injections, even of particulate steroids.
Accordingly, there is a need for steroid formulations with longer local retention and better symptom relief after ESI. The duration and effectiveness of ESI have usually been assessed by questionnaires on patients’ symptoms, but the residual amount of steroid in the tissue has not actually been measured. Thus, for quantitative comparison of steroid retention and development of more effective formulations, an adequate animal model is essential. However, to the best of our knowledge, there exists no such animal model.
In the present study, we designed a rabbit model for performing ESI under fluoroscopic guidance, simulating actual practice. Using this model, we can measure the locally retained steroid amount, which could help determine the duration for which the steroid remained in the injected area, as well as its spread through the epidural space. Therefore, the purpose of this study was to develop a rabbit ESI model for analyzing steroid retention in the tissue, and to assess the difference in steroid retention in the model according to the location and time elapsed after ESI.
Our study was performed from March 2017 to December 2017. All procedures were approved by the Animal Care and Use Committees of Seoul National University Bundang Hospital (approval number: BA1608-206/050-01).
Thirteen female New Zealand white rabbits (weight range, 3.1–3.5 kg at the beginning of the study) were obtained from DooYeol Biotech (Seoul, Korea) and Orient Bio (Seongnam, Korea). Rabbits were housed in individual cages with a controlled light cycle (12-hr light/12-hr dark cycle) and temperature (21°C ± 2°C). Standard laboratory chow and tap water were available ad libitum.
Twelve of the rabbits were allocated to three different groups according to time of sacrifice: 3, 7, and 15 days after ESI (Fig. 1). The remaining rabbit was sacrificed immediately after ESI to obtain baseline data. At each time-point, the rabbits were sacrificed, and two lumbar spinal segments were surgically harvested: the lowest two lumbar vertebrae as well as the two lumbar vertebrae immediately above these.
A radiologist performed ESI under fluoroscopic guidance, in the manner used in actual clinical practice. Rabbits were anesthetized with intramuscular injection of alfaxalone (Alfaxan, 10 mg/mL; Jurox Pty, Ltd., Rutherford, Australia; 5 mg/kg body weight) and xylazine (Rompun, 23.32 mg/mL; Bayer Korea, Ansan, Korea; 5 mg/kg body weight). Subsequently, the rabbit was placed in the prone position on the fluoroscopy procedure table (Fig. 2A). We selected the interlaminar trajectory between the lowest two lumbar spine segments for epidural injection. A 25-gauge spinal Quincke needle was used, and appropriate needle tip placement was confirmed by fluoroscopy (Fig. 2B) with the injection of approximately 0.5 mL of contrast material (iohexol, Omnipaque 300, 300 mg iodine per mL; GE Healthcare Co., Ltd., Shanghai, China) (Fig. 2C). The injectate was 40 mg (1 mL) triamcinolone acetonide suspension (TA; Triam, 40 mg/mL; Shinpoong Pharmaceuticals, Seoul, Korea) and the injection rate was approximately 0.3 mL/sec. A fluoroscopic image was obtained following the injection, to demonstrate the washout of the contrast agent.
Rabbits were sacrificed after induction of anesthesia, using potassium chloride (150 mg/mL; JW Pharmaceutical, Seoul, Korea; 150 mg/kg body weight). A longitudinal incision was made along the midline of the prone-positioned rabbit. The level of the tissue harvested was confirmed by palpation of the lumbosacral junction and spinous process of the lumbar vertebra. The paraspinal muscle was incised transversely using a sharp scalpel, and a rongeur was used for dividing the lumbar bony segments. The dorsal section of the rabbit containing the lumbar spine was excised en bloc (Fig. 3).
Collected spinal columns were sent to an analytical chemistry laboratory (Biological Mass Spectrometry Group at the Dankook University, Cheonan, Korea) for measuring the residual steroid amount (RSA) and residual steroid concentration (RSC) in the tissue, using a validated high-performance liquid chromatography-mass spectrometry/mass spectrometry (HPLC-MS/MS) assay. To extract TA from the tissue, the whole excised spine tissue was soaked in 50 mL of methanol and agitated in a shaking incubator for 12 hours. The extracted solution (1 mL) was diluted 50 times with methanol and then centrifuged for 10 minutes at 14,000 rpm. The supernatant (10 μL) was injected into an HPLC-MS/MS system (LC-20 Prominence HPLC system; Shimadzu, Tokyo, Japan) and an API 2000 triple quadrupole mass spectrometer (AB/SCIEX, Poster City, CA). The chromatographic separation was performed using a Phenomenex Luna C18 column (2.0 × 150 mm, 5-μm particle size). The mobile phase consisted of 0.1% formic acid in water and acetonitrile at a volume ratio of 50:50, with a flow rate of 0.25 mL/min.
The steroidal compound eluted from the column was transferred into the MS/MS instrument with an electrospray ionization source in positive ion mode. The gas temperature was 400°C, the ion spray voltage was 5,500 V, and the pressures of curtain and collision gases were 16 and 6 psi, respectively. The TA was monitored using multiple reaction monitoring, with the m/z transition of 435.1 to 415.0. For further TA confirmation, additional transitions (435.1 to 171.4 and 435.1 to 397.1) were also moni-tored. The assay exhibited excellent linearity (R2 value of 0.9917) over a TA concentration range of 100–600 ng/mL.
The RSA and RSC in the lumbar vertebrae were compared between the extracted upper and lower segments. These measurements were also compared with respect to the day of harvest. To this end, all comparisons were performed using the linear mixed-effects model, which accounts for two-way measurements per rabbit. For the mixed-effects model, the level of the segments (upper or lower) and the day of harvest (3, 7, or 15) were used as fixed effects, and rabbit identification was used as a random effect. Wilcoxon’s signed-rank test was used as the post hoc test for comparing RSA and RSC on each harvest day. Statistical analysis was performed using the software Stata ver. 14.0 (StataCorp LLC, College Station, TX); P < 0.05 was considered statistically significant.
The 12 rabbits in the experimental groups were able to bear weight, and exhibited normal gait after the ESI procedure; the rabbit used for obtaining baseline data was not assessed as it was sacrificed immediately after ESI.
The collected spinal segments weighed 48.6 ± 9.07 mg (mean ± standard deviation). Mean RSA/RSC values in the immediately collected tissues were 1.3 mg per 19.4 ppm for the injected lower segment and 0.93 mg per 20.9 ppm for the adjacent upper segment.
The RSA values in the injected area versus the adjacent area (mean, mg) on each day of sacrifice, and the percentage normalized to the immediately collected tissue, were as follows: 17 × 10−2 (13%) vs. 4.7 × 10−2 (5.0%), 7.9 × 10−2 (6.0%) vs. 2.4 × 10−2 (2.6%), and 2.5 × 10−3 (0.25%) vs. 0.65 × 10−3 (0.070%) for sacrifice after 3, 7, and 15 days, respectively.
The RSC values in the injected area versus the adjacent area (mean, ppm) on each day of sacrifice, and the percentage normalized to the immediately collected tissue, were as follows: 3.3 (17%) vs. 1.0 (4.8%), 2.1 (11%) vs. 0.56 (2.7%), and 0.048 (0.25%) vs. 0.015 (0.071%) for sacrifice after 3, 7, and 15 days, respectively (Table 1). The RSA and RSC values in the injected segment were significantly greater than those in the adjacent segment (P < 0.001, both). The RSA and RSC significantly decreased over time (P = 0.009 and P = 0.016, respectively) (Fig. 4).
In this study, we developed a rabbit model that was used to assess the amount of steroid remaining after fluoroscopy-guided lumbar ESI. As expected, the RSA and RSC of the injected segments were significantly larger than those of the adjacent segments; these results assure the reliability of this rabbit ESI model. We also found that the injected TA remained in the harvested tissue for 15 days after lumbar ESI. The amount and concentration of the remaining steroid decreased over time, and only 2.5 × 10−3 mg of TA remained in the injected segment by 15 days after ESI. This suggests that the injected steroid is dispersed or degraded relatively rapidly. These results are compatible with previous reports that ESI shows robust effectiveness in providing short-term symptom relief to patients with disc herniation [4] and those with spinal stenosis exhibiting neurogenic claudication or radiculopathy [7]. However, for long-term effectiveness, the evidence was conflicting or less reliable for both patient groups [4,7].
In this study, TA, a particulate steroid, was used instead of a nonparticulate steroid, such as dexamethasone. Particulate agents have been shown to be superior to nonparticulate agents in terms of the duration of pain relief [6,8,9]; however, there are reports on serious adverse events after particulate steroid administration, such as spinal cord infarction or cerebellar infarction [10,11], presumably due to particle embolization. The current guideline of the US Food and Drug Administration [12] recommends against using particulate formulations in ESI. We believe that animal studies are crucial for the development of longer-lasting and safe particulate steroid formulations with more pain relief, and therefore, used TA in the development of the rabbit ESI model.
The epidural space is encased between the dura mater and the walls of the vertebral canal, with intermittent attachment via connective tissue. There is a thin layer of areolar tissue embracing the vertebral venous plexus and epidural adipose tissue, which lies in a recess between the dura mater and the ligament flava [13,14]. Drugs injected into the epidural space are absorbed and stored in the epidural fat, particularly lipophilic drugs, such as steroids. Redistribution of these drugs is affected by lipophilicity, tissue permeability, local blood supply, and the surface area involved [15].
Madsen et al. [16] reported that 1.0 mL of contrast agent, which was injected through an epidural catheter at the level of the 6th–7th lumbar vertebrae, is distributed to the level of the 8th–9th thoracic vertebrae in a rabbit model. Our study showed similar distribution of the contrast agent, and only 1.3 mg of injected TA (40 mg) remained at the injected segment. However, the amount of steroid retained in the injected level was significantly larger than that in the adjacent level. This finding could be explained by the characteristics of steroids. In contrast to water-soluble contrast agents, which might spread promptly to other spinal levels through the potential epidural space, lipophilic steroid agents might be dissolved in the epidural adipose tissue of the injected level. This could imply that targeted injection at the specific spinal level may be more effective.
The first documented steroid injection into the epidural space for managing lumbar radicular pain was performed in 1952 [17]. Since then, the epidural administration of steroids has been one of the best-studied subjects in interventional pain management. Although Beall et al. [18] reported the tissue distribution of clonidine after intraforaminal implantation of biodegradable pellets, a methodology for investigating the presence of corticosteroid residue in the tissue after ESI has not been reported. In the present study, we designed a rabbit model to measure the quantity of drug remaining after ESI, over time. This rabbit model will make it possible to compare the residual concentrations of various steroid formulations.
There are several limitations to our experimental study. First, the number of rabbits in each group and the total number of animals used were relatively small. Further studies with larger sample sizes would strengthen our results. Second, the measurement method could not differentiate between residual amounts of steroid in specific tissues, such as the spinal cord, dura mater, epidural fat, or bones. However, the mechanism of the lumbar pain relief provided by steroids is complex [19], and investigating the RSA in specific tissues might be meaningless. Third, we only used the midline approach at the lowest lumbar level for the ESI, for experimental convenience. Other experiments with different approaches, such as transforaminal routes or different injection levels, are war-ranted. Fourth, the amount of injectate was relatively large for the rabbit. Kim et al. [20] reported the usual TA dose of ESI in adult patients is approximate 20 mg. Because this study aimed to develop rabbit ESI model and to analyze steroid retention in the model, we used sufficient amount of steroid to assess the analytical feasibility of residual steroid in the harvested tissue. In addition, given that TA is a non-solution suspension, it was assumed that injecting the entire volume of the vial (40 mg) is more consistent than using the vial fraction.
In conclusion, we verified that retention of injected steroids can be quantitatively assessed using the rabbit ESI model; the duration of retention and spread of the injected steroid can also be analyzed. Thus, this model could be useful not only for comparing current steroid medications, but also for developing new, better steroid formulations.
ACKNOWLEDGMENTS
The authors thank the Medical Research Collaborating Center at Seoul National University Bundang Hospital for performing statistical analyses.
This work was supported by a grant from Mid-career Researcher Program through the National Research Foundation of Korea (Ministry of Science and ICT, grant number: NRF-2016R1A2B4010992) and by a grant from the Seoul National University Bundang Hospital Research Fund (grant number: 14-2014-027).
Notes
REFERENCES
1. Jacobs JM, Hammerman-Rozenberg R, Cohen A, Stessman J. Chronic back pain among the elderly: prevalence, associations, and predictors. Spine (Phila Pa 1976). 2006; 31:E203–7. DOI: 10.1097/01.brs.0000206367.57918.3c. PMID: 16582841.

2. Manchikanti L, Pampati V, Falco FJ, Hirsch JA. An updated assessment of utilization of interventional pain management techniques in the Medicare population: 2000 – 2013. Pain Physician. 2015; 18:E115–27. PMID: 25794210.
3. Chou R, Loeser JD, Owens DK, Rosenquist RW, Atlas SJ, Baisden J, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009; 34:1066–77. DOI: 10.1097/BRS.0b013e3181a1390d. PMID: 19363457.
4. Manchikanti L, Benyamin RM, Falco FJ, Kaye AD, Hirsch JA. Do epidural injections provide short- and long-term relief for lumbar disc herniation? A systematic review. Clin Orthop Relat Res. 2015; 473:1940–56. DOI: 10.1007/s11999-014-3490-4. PMID: 24515404. PMCID: PMC4419020.

5. Friedly JL, Comstock BA, Turner JA, Heagerty PJ, Deyo RA, Sullivan SD, et al. A randomized trial of epidural glucocorticoid injections for spinal stenosis. N Engl J Med. 2014; 371:11–21. DOI: 10.1056/NEJMoa1313265. PMID: 24988555.

6. Kennedy DJ, Plastaras C, Casey E, Visco CJ, Rittenberg JD, Conrad B, et al. Comparative effectiveness of lumbar transforaminal epidural steroid injections with particulate versus nonparticulate corticosteroids for lumbar radicular pain due to intervertebral disc herniation: a prospective, randomized, double-blind trial. Pain Med. 2014; 15:548–55. DOI: 10.1111/pme.12325. PMID: 24393129.

7. Kreiner DS, Shaffer WO, Baisden JL, Gilbert TJ, Summers JT, Toton JF, et al. North American Spine Society. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update). Spine J. 2013; 13:734–43. DOI: 10.1016/j.spinee.2012.11.059. PMID: 23830297.

8. Kim JY, Lee JW, Lee GY, Lee E, Yoon CJ, Kang HS. Comparative effectiveness of lumbar epidural steroid injections using particulate vs. non-particulate steroid: an intra-individual comparative study. Skeletal Radiol. 2016; 45:169–76. DOI: 10.1007/s00256-015-2277-3. PMID: 26537154.

9. Lee JW, Park KW, Chung SK, Yeom JS, Kim KJ, Kim HJ, et al. Cervical transforaminal epidural steroid injection for the management of cervical radiculopathy: a comparative study of particulate versus non-particulate steroids. Skeletal Radiol. 2009; 38:1077–82. DOI: 10.1007/s00256-009-0735-5. PMID: 19543892.

10. Suresh S, Berman J, Connell DA. Cerebellar and brainstem infarction as a complication of CT-guided transforaminal cervical nerve root block. Skeletal Radiol. 2007; 36:449–52. DOI: 10.1007/s00256-006-0215-0. PMID: 17216270.

11. Wybier M, Gaudart S, Petrover D, Houdart E, Laredo JD. Paraplegia complicating selective steroid injections of the lumbar spine. Report of five cases and review of the literature. Eur Radiol. 2010; 20:181–9. DOI: 10.1007/s00330-009-1539-7. PMID: 19680658.

12. Benzon HT, Huntoon MA, Rathmell JP. Improving the safety of epidural steroid injections. JAMA. 2015; 313:1713–4. DOI: 10.1001/jama.2015.2912. PMID: 25822848.

13. Parkin IG, Harrison GR. The topographical anatomy of the lumbar epidural space. J Anat. 1985; 141:211–7. PMID: 4077717. PMCID: PMC1166402.
14. Qureshi AI, Qureshi MH, Malik AA, Khan AA, Sohail A, Saed A, et al. Catheter-based transepidural approach to cervical and thoracic posterior and perineural epidural spaces: a cadaveric feasibility study. J Vasc Interv Neurol. 2015; 8:43–9. PMID: 26060530. PMCID: PMC4445339.
15. Reina MA, Franco CD, López A, Dé Andrés JA, van Zundert A. Clinical implications of epidural fat in the spinal canal. A scanning electron microscopic study. Acta Anaesthesiol Belg. 2009; 60:7–17. PMID: 19459550.
16. Madsen JB, Jensen FM, Faber T, Bille-Hansen V. Chronic catheterization of the epidural space in rabbits: a model for behavioural and histopathological studies. Examination of meptazinol neurotoxicity. Acta Anaesthesiol Scand. 1993; 37:307–13. DOI: 10.1111/j.1399-6576.1993.tb03720.x. PMID: 8517109.

17. Robecchi A, Capra R. Hydrocortisone (compound F); first clinical experiments in the field of rheumatology. Minerva Med. 1952; 43:1259–63. PMID: 13036739.
18. Beall DP, Deer TR, Wilsey JT, Walsh AJ, Block JH, McKay WF, et al. Tissue distribution of clonidine following intraforaminal implantation of biodegradable pellets: potential alternative to epidural steroid for radiculopathy. Pain Physician. 2012; 15:E701–10. PMID: 22996864.

19. Baqai A, Bal R. The mechanism of action and side effects of epidural steroids. Tech Reg Anesth Pain Manag. 2009; 13:205–11. DOI: 10.1053/j.trap.2009.06.009.

20. Kim EJ, Moon JY, Park KS, Yoo da H, Kim YC, Sim WS, et al. Epidural steroid injection in Korean pain physicians: a national survey. Korean J Pain. 2014; 27:35–42. DOI: 10.3344/kjp.2014.27.1.35. PMID: 24478899. PMCID: PMC3903799.

Fig. 2
Epidural injection of triamcinolone through the interlaminar route. (A) Spine anteroposterior radiograph of the prone-positioned rabbit. (B) Interlaminar approach with a 25-gauge spinal Quincke needle. (C) Appropriate needle tip placement confirmed by the injection of a contrast agent.

Fig. 3
Representative image showing the tissue being harvested. Two dorsal sections of the rabbit corresponding to the lumbar spine were excised en bloc, including the lowest two (level of epidural steroid injection) lumbar vertebrae and the segment immediately above them.
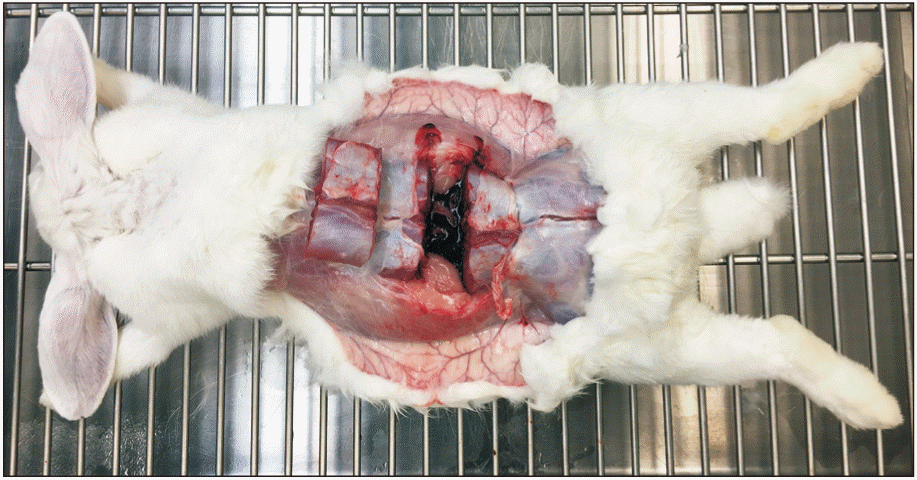
Fig. 4
Line graphs of residual steroid measurements in the harvested tissue. (A) Residual steroid amount (RSA). (B) Residual steroid concentration (RSC).
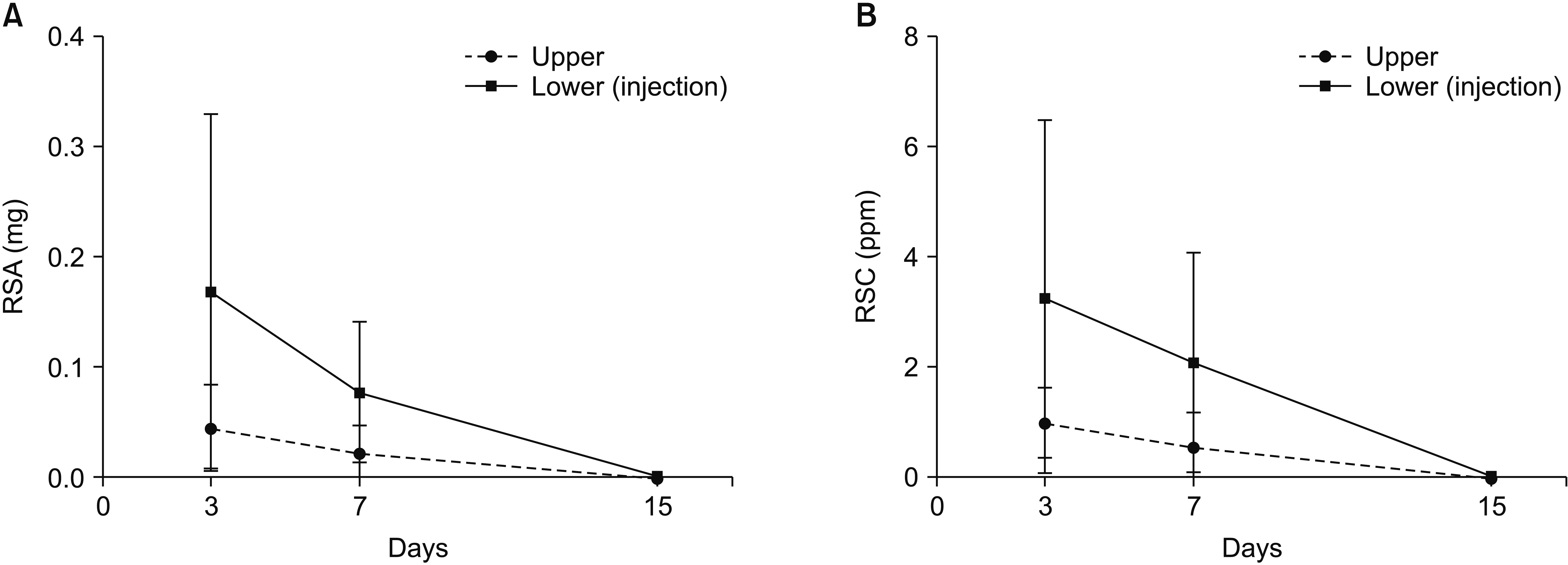
Table 1
Comparison of RSA and RSC
| Variable | RSA (mg) | RSC (ppm) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Upper | Lower | P value | Upper | Lower | P value | |
| 3 days | 4.7 × 10−2 ± 3.1 × 10−2 | 1.7 × 10−1 ± 1.6 × 10−1 | 0.002*a | 1.0 ± 0.63 | 3.3 ± 3.2 | 0.005*a |
| 7 days | 2.4 × 10−2 ± 2.6 × 10−2 | 7.9 × 10−2 ± 6.3 × 10−2 | 0.002*a | 0.56 ± 0.64 | 2.1 ± 2.0 | 0.002*a |
| 15 days | 0.65 × 10−3 ± 0.69 × 10−3 | 2.5 × 10−3 ± 2.7 × 10−3 | 0.002*a | 0.015 ± 0.016 | 0.05 ± 0.05 | 0.002*a |
| LMM | 0.009* | 0.016* | ||||




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download