Abstract
Background and Objectives
Liver cirrhosis is accompanied by abnormal vascular shunts. The Wnt pathway is essential for endothelial cell survival and proliferation. C-reactive protein (CRP), which is produced by hepatocyte, activates angiogenesis in cardiovascular diseases.
Methods and Results
The expression of CRP in CCl4-injured rat livers was detected using qRT-PCR and Western blotting after transplantation of placenta-derived mesenchymal stem cells (PD-MSCs) into rats. To determine whether CRP functions in hepatic regeneration by promoting angiogenesis through the Wnt pathway, we detected VEGF and β-catenin in liver tissues and BrdU and β-catenin in hepatocytes by immunofluorescence. The expression levels of CRP, Wnt pathway-related and angiogenic factors were increased in CCl4-injured and PD-MSCs transplanted rat livers. In vitro, the expression levels of Wnt signaling and angiogenic factors were decreased in siRNA-CRP-transfected rat hepatocytes.
The liver is an essential organ for the metabolism for lipids, proteins and carbohydrates, as well as the detoxification for endogenous metabolites (1). Liver sinusoidal endothelial cells (LSECs), which compose the hepatic vasculature, are in charge of important functions such as the proliferation of hepatocytes in the liver (2). LSECs releases angiocrine, including Wnt2 and hepatic growth factor (HGF), before the activation of angiogenesis and stimulate the regeneration of hepatocytes (3). Angiocrine signals induced by LSECs regulate the balance between fibrosis and hepatic regeneration (4). Hepatic failure involves diffused fibrosis, abnormal lobular architecture and the formation of intrahepatic vascular shunts (5). Therefore, vascular remodeling in liver tissues is a critical target for treating hepatic failure.
C-reactive protein (CRP) is known as the acute phase protein that is produced and released by hepatocytes in response to infection or tissue damage (6). CRP can be classified as monomeric CRP (mCRP) and pentameric CRP (pCRP) (7). Related to these isoforms of CRP, several studies have demonstrated pro-inflammatory and anti-inflammatory effects of pCRP and mCRP, respectively (8). Recently, it has been shown that mCRP upregulates the tube-like formation signaling pathway in endothelial cells by activating protein C-ets-1 (ETS1), transcription factor and phosphorylating AKT, thereby increasing the release of chemokine ligand 2 (CCL2) (9). Additionally, together with oxidized low-density lipoprotein (oxLDL), CRP decelerates and stabilizes atherosclerotic progression by reducing the secretion of inflammatory factors such as TNF-α by macrophages (10). However, the regenerative effect of CRP has not yet been demonstrated in hepatic failure.
In the Wnt signaling, β-catenin is a key factor regulating transcriptional activity (11). Some studies have demonstrated that the inhibition of the Wnt pathway in the liver leads to pathophysiological events (12). The β-catenin-dependent Wnt pathway is essential for EC survival and proliferation as well as the regulation of endothelial permeability (13). Accumulated evidence shows that endothelial β-catenin affects tight junctions, resulting in vascular integrity (14). However, the effect of β-catenin related to vascular remodeling in liver cirrhosis is not yet clear.
Placenta-derived mesenchymal stem cells (PD-MSCs) have been characterized and have come to focus in stem cell research because of their proliferation, self-renewal, and low immunogenicity (15). In particular, transplanted PD-MSCs have been demonstrated to ameliorate CCl4-in-duced liver cirrhosis in mice (16). We previously reported that PD-MSCs have the ability to promote regeneration of hepatocyte via autophagic mechanism as well as induce IL-6/STAT3 signaling by reducing promoter methylation, further promoting the proliferation of hepatocytes in a CCl4-injured liver (17). Additionally, we demonstrated decreased CRP expression induces abnormal vascular structure in bile duct ligation rat model (18). However, the fundamental mechanism on vascular remodeling by CRP and their therapeutic potentials on hepatic diseases depend on PD-MSCs transplantation are still unclear.
We therefore aimed to investigate the expression of CRP, β-catenin and angiogenic factors in a CCl4-injured rat liver model by PD-MSC transplantation and determine the regenerative effect of CRP through Wnt signaling and angiogenesis.
Six-week-old Sprague-Dawley rats were obtained (Orient Bio Inc.) and maintained in an air-conditioned facility. Hepatic failure was induced CCl4 (approximately 1.6 g/kg; Sigma-Aldrich) dissolved at 0.8 mg/ml in corn oil and intraperitoneally injected at 0.2 ml/100 g body weight twice a week for 3 weeks. Control rats (n=5) were injected with an equal volume of corn oil alone. PD-MSCs (2×106 cells, 8∼10 passages) were injected into the tail vein of rats (TTX; n=19). Non-transplanted (NTX; n=19) rats were maintained as sham controls. Liver tissues were harvested at 1, 2 and 3 weeks from rats in all groups. All animal experimental processes and the experimental protocol were approved by the Institutional Animal Care and Use Committee of CHA University, Seongnam, Korea (IACUC-140009).
Equal amounts of serum from individual animals were pooled (n=5). To isolate exosomes from rat serum, we used ExoQuick Exosome Precipitation solution (System Biosciences). The solution for exosome precipitation was used according to the manufacturer’s instructions.
All participants provided written and informed consent prior to sample collection. Placental tissues were collected from women who had no medical, obstetrical, and surgical complications and who delivered at term (37 gestational weeks). The collection of samples and their use for research purposes were approved by the IRB of CHA General Hospital, Seoul, Korea (IRB 07-18). PD-MSCs were harvested as previously described. PD-MSCs were collected from the inner side of the chorionic membrane of the placenta. The cells scraped from the membrane were treated with 0.5% collagenase IV (Sigma-Aldrich) and cultured in Ham’s F-12/Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 1% penicillin/streptomycin (Pen-Strep, Gibco), 25 ng/ml human fibroblast growth factor-4 (Peprotech), and 100 μg/ml heparin (Sigma-Aldrich). Rat epithelial cells (WB-F344s) were cultured in α-MEM (Gibco) supplemented with 1% Pen-Strep and 5% FBS. Human umbilical vein endothelial cells (HUVECs) were cultured in endothelial cell growth medium (EGM)-2 SingleQuots (Lonza) at 37℃ in a 5% CO2 incubator.
WB-F344s (2×105) and HUVECs (6×104) were seeded onto cover glasses (Marlenfeld GmbH & Co.), and for double cell co-culture experiments, WB-F344s (2×105) were seeded onto cover glasses in a 100 mm culture plate (Corning). After 3 hours, the cells were exposed to 3 mM CCl4 for 24 hours. The next day, the cells on cover glass were transferred to 6-well plates and co-cultured with PD-MSCs (6×104) were in Transwell inserts (3 μm pore).
To sort co-cultured WB-F344s and HUVECs, we performed MACS analysis. Harvested WB-F344s and HUVECs were incubated with biotin-conjugated anti-CD31 (Miltenyi Biotec Inc.). Anti-biotin microbeads (Miltenyi Biotec Inc.) were reacted with the collected cells. The bound cells were sorted using MidiMACS Starting Kits (Miltenyi Biotec Inc.).
Total RNA was isolated from rat liver tissues and cells using TRIzol (Invitrogen). Reverse transcription was performed with 500 ng total RNA and Superscript III reverse transcriptase (Invitrogen). Real-time PCR was performed using SYBR EX Taq (Roche) and an ExicyclerTM 96 quantitative thermal block (Bioneer). PCR conditions were as follows: denaturation at 95℃ for 15 minutes and 20 seconds; 40 cycles at 95℃ for 30 seconds; annealing at 52∼60℃ for 40 seconds; extension at 70℃ for 15 minutes; and a final extension at 72℃ for 7 minutes. Gene expression was normalized to the expression of GAPDH. The sequences of the primers are as follows: rat CRP forward 5’- GCT TTT GGT CAT GAA GAC ATG TC -3’, rat CRP reverse 5’- TCA CAT CAG CGT GGG CAT AG -3’, rat Axin2 forward 5’- AAA CCT ATG CCT GTC TCC TC -3’, rat Axin2 forward 5’- ATC CAC ACA TTT CTC CCT CT -3’, rat VEGF forward 5’- ACG GAC AGA CAG ACA GAC AC -3’, rat VEGF reverse 5’- CTT CTG GGC TCT TCT TCT CTC TC -3’, rat albumin forward 5’- GCC CCA GAA CTC CTT TAC TA -3’, rat albumin reverse 5’- AAT CTC TGC ATA CTG GAG CA -3’, rat GAPDH forward 5’- TCC CTC AAG ATT GTC AGC AA -3’, rat GAPDH reverse 5’- AGA TCC ACA ACG GAT ACA TT -3’, human CRP forward 5’- TCG TAT GCC ACC AAG AGA CAA GAC A -3’, human CRP reverse 5’- AAC ACT TCG CCT TGC ACT TCA TAC T -3’, human Axin2 forward 5’- TCC CCA CCT TGA ATG AAG AA -3’, human Axin2 reverse 5’- TGG TGG CTG GTG CAA AGA -3’, human VEGF forward 5’- GCC TTG CCT TGC TGC TCT AC -3’, human VEGF reverse 5’- ACA TCC ATG AAC TTC ACC ACT TCG -3’, human albumin forward 5’- TGA GAA AAC GCC AGT AAG TGA C -3’, human albumin reverse 5’- TGC GAA ATC ATC CAT AAC AGC -3’, human GAPDH forward 5’- GCA CCG TCA AGG CTG AGA AC -3’, and human GAPDH reverse 5’- GTG GTG AAG ACG CCA GTG GA -3’. All reactions were conducted in duplicate or triplicate. Relative mRNA expression was analyzed by the comparative CT method.
Rat liver tissues and cells were lysed in RIPA buffer (Sigma-Aldrich) supplemented with protease inhibitor cocktail (Roche) and phosphatase inhibitor (Sigma-Aldrich). A total of 45 μg protein was separated in sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE). The separated proteins were transferred onto PVDF membranes (Bio-Rad). The membranes were incubated with anti-CRP (1:1,000, Abcam Inc.), anti-VEGF (R&D system), anti-VEGFR2 (R&D system), anti-β-catenin (active form, Cell Signaling), anti-pGSK3 (Cell Signaling), anti-β-catenin (active form, Cell Signaling), anti-albumin (Novus), anti-HNF1α (Abcam Inc.), anti-cyclinD1 (AbFrontier), anti-actin (Sigma-Aldrich), anti-tubulin (Abcam Inc.), and anti-GAPDH (AbFrontier) antibodies at 4℃ over-night. The membranes were then incubated with horseradish peroxidase-conjugated secondary anti-mouse IgG (Cell Signaling), anti-rabbit IgG (Cell Signaling) or anti-goat IgG (Santa Cruz) antibody for 1 hour at RT. Bands were detected using Clarity Western ECL kit (Bio-Rad).
To observe the degree of inflammation in tissues following transplantation with PD-MSCs or not (NTX), we used anti-NF-κB antibody. The sections were incubated in 3% H2O2 in methanol to block endogenous peroxidase activity. After antigen retrieval, the slides were incubated with an anti-NF-κB antibody (Santa Cruz) at 4℃ over-night, followed by an hour incubation with biotinylated secondary anti-rabbit antibody at room temperature (RT). Incubation with horseradish peroxidase-conjugated streptavidin–biotin complex (Dako) and 3,3-diaminobenzidine (EnVision Systems) was performed to generate a chromatic signal. The samples were counterstained with Mayer’s hematoxylin. Additionally, the percentage of hepatocytes with NF-κB-positive nuclei relative to the total number of hepato-cytes was counted in randomly selected sections (three fields per rat at ×400 magnification). Images were detected using a Zeiss Axioskop 2 MAT microscope (Carl Zeiss MicroImaging).
To analyze the localization of VEGF and active β-ca-tenin in liver tissues, we embedded rat livers in Tissue-Tek OCT compound (Sakura Finetechnical Co. Ltd.) and stored at −80℃. Cryosections of tissues were fixed in methanol (MERCK) and incubated in blocking solution (Dako). Anti-VEGF (Novus) and anti-active β-catenin (Cell Sig-naling) antibodies were used as primary antibodies at 4℃ over-night. The secondary antibodies, Alexa 488 and 569 (Invitrogen), were incubated.
To analyze the localization of β-catenin and BrdU in rat hepatocytes, the WB-F344s co-cultured with PD-MSCs were fixed with 4% paraformaldehyde and permeabilized with 0.25% Triton X-100 and 3% H2O2. The cells were incubated with blocking solution (Dako) at RT and rabbit anti-β-catenin (Cell Signaling) and mouse anti-BrdU (Cell Signaling) antibodies at 4℃ over-night. Subsequently, the cells were incubated with Alexa 568- or 488-conjugated secondary antibodies (Invitrogen) at RT. The slides were counterstained with DAPI. The images were observed with a fluorescence microscope EVOS (Thermo Fisher Scientific). All experiments were performed in triplicate.
The concentrations of interleukin (IL)-6, IL-10 and CRP were determined by enzyme-linked immunosorbent assay (ELISA). Equal amounts of serum from individual animals or culture media were pooled (n=5). All reactions were performed in triplicate. IL-6 and IL-10 concentrations were assayed using rat IL-6 and IL-10 ELISA kits (R&D system), according to the manufacturer’s protocols. The concentration of CRP was measured using a rat C-Reactive Protein ELISA kit (Invitrogen) according to the manufacturer’s instructions. The experiments were performed in triplicate.
Statistical significance was analyzed using Student’s t-test with a significance level of p<0.05, and data were represented as the mean±standard deviation (SD). For multivariate data analysis, group differences were assessed with two-way ANOVA, followed by Tukey HSD test. All experiments were conducted in duplicate or triplicate.
To evaluate the anti-inflammatory effects of PD-MSC transplantation, we analyzed NF-κB expression in CCl4-injured rat livers using immunohistochemistry. As shown in Fig. 1A, in the CCl4-damaged NTX group, NF-κB was excessively localized in the nuclei for up to 3 weeks; however, transplantation of PD-MSCs resulted in a marked reduction in NF-κB localization. Moreover, the number of NF-κB-positive cell was significantly lower in the TTX group than in the NTX group (*, p<0.05, Fig. 1B). Furthermore, we assayed the concentrations of IL-10, an anti-inflammatory factor, and IL-6, a regeneration factor, in the rat serum via ELISA. Compared to that in the NTX group, the expression of IL-10 in the rat serum was significantly increased in the TTX group (*, p<0.05, Fig. 1C). The secreted form of IL-6, which functions in hepatic regeneration, was also significantly increased in the TTX group (*, p<0.05, Fig. 1D).
Similar to IL-10 and IL-6, the expression of CRP in serum (Fig. 2A) and total liver protein (Fig. 2C) progressively increased in the TTX group compared to that in the control and NTX groups of in the CCl4-injured rat model. Additionally, CRP in exosomes isolated from serum was upregulated in the TTX group when normalized to heat shock protein (HSP)-70 and HSP-90, which are internal markers of exosomes (Fig. 2B and 2D). Therefore, PD-MSCs attenuate inflammatory responses and promote anti-inflammation through the induction of CRP expre-ssion.
To analyze the correlation between Wnt signaling and angiogenesis, we confirmed the expression and localiza-tion of the factors related to angiogenesis and Wnt sig-naling. Compared to those in the control and NTX groups, the factors related to angiogenesis, such as VEGF and VEGFR2, were significantly induced in the TTX group (*, p<0.05, Fig. 3A, Supplementary Fig. S1A, and S1C). However, the expression of pGSK3, a Wnt inhibitory molecule, was significantly decreased in the TTX group compared to that in the control and NTX groups (*, p<0.05, Fig. 3B, Supplementary Fig. S1B). In contrast to GSK3, the active form of β-catenin was upregulated by PD-MSCs in CCl4-injured rat livers (Fig. 3B, Supplementary Fig. S1D). To investigate the localization and expression of VEGF and β-catenin, we performed immunofluorescence. VEGF and active β-catenin were localized in the cytoplasm and nuclei, respectively. Additionally, the expre-ssion of VEGF and β-catenin was higher in the control and TTX groups than in the NTX group (Fig. 3C). These results mean that activated β-catenin by PD-MSCs transplantation translocates into nucleus following promotes expression of VEGF in CCl4-injured rat livers. Further, to demonstrate the interaction between CRP, Wnt signaling, and angiogenesis in the liver, we analyzed the correlation between CRP, β-catenin, and VEGF of normalized fold change by band intensity of western blot. The correlation coefficient (R2) of CRP and β-catenin was 0.8992 (Fig. 3D) as well as that of β-catenin and VEGF was 0.9295 (Fig. 3E). Therefore, these results represent that CRP, Wnt pathway, and vascular remodeling strongly correlate with one another in CCl4-injured rat liver.
To demonstrate the correlation among CRP, Wnt, and angiogenic and hepatic regeneration factors in hepato-cytes, we transfected siRNA-CRP in rat hepatocytes and analyzed the mRNA and protein levels of CRP, albumin and factors related to Wnt signaling and angiogenesis. The expression of CRP, Axin2 (Fig. 4B), VEGF, and albumin (ALB) (Supplementary Fig. S2) mRNA was lower in siRNA-CRP-transfected hepatocytes than in non-transfected hepatocytes. As assessed by western blotting, CRP and VEGF protein expression was significantly suppressed via siRNA-CRP transfection (*, p<0.05, Fig. 4A and 4B). Additionally, the expression of β-catenin, a key factor in the Wnt pathway, was significantly inhibited by siRNA-CRP transfection in rat hepatocytes (*, p<0.05, Fig. 4C). Also, hepatic regeneration and proliferation factors such as ALB, hepatic nuclear factor 1 alpha (HNF1α), and cyclinD1 were significantly repressed by siRNA-CRP transfection in WB-F344s (*, p<0.05, Fig. 4D∼F). Therefore, CRP directly regulates angiogenesis through the Wnt pathway and promotes hepatic regeneration.
To demonstrate the effect of CRP induced by PD-MSCs, we cultured CCl4-treated rat hepatocytes with PD-MSCs. The expression of CRP at both mRNA and protein levels was upregulated in rat hepatocytes by co-culture with PD-MSCs (Fig. 5A and 5B). To verify cell proliferation and localization of β-catenin, we performed immunofluorescence staining for BrdU and active β-ca-tenin in rat hepatocytes that were co-cultured with PD-MSCs post-CCl4 treatment. The localization of BrdU and β-catenin in rat hepatocytes is shown in Fig. 5C. The number of BrdU- or β-catenin-positive cells was significantly higher in the CCl4-treated and PD-MSC co-cultured group than in the non-co-cultured group (*, p<0.05, Fig. 5D). Also, the protein level of HNF1α and cyclinD1, hepatic regeneration and proliferation markers, was dramatically decreased in CCl4 treated WB-F344s while their expression were recovered by PD-MSCs co-cultivation (*, p<0.05, Fig. 5E∼G). Therefore, CRP upregulation by PD-MSCs in rat hepatocytes activates Wnt signaling and facilitates hepatocyte regeneration.
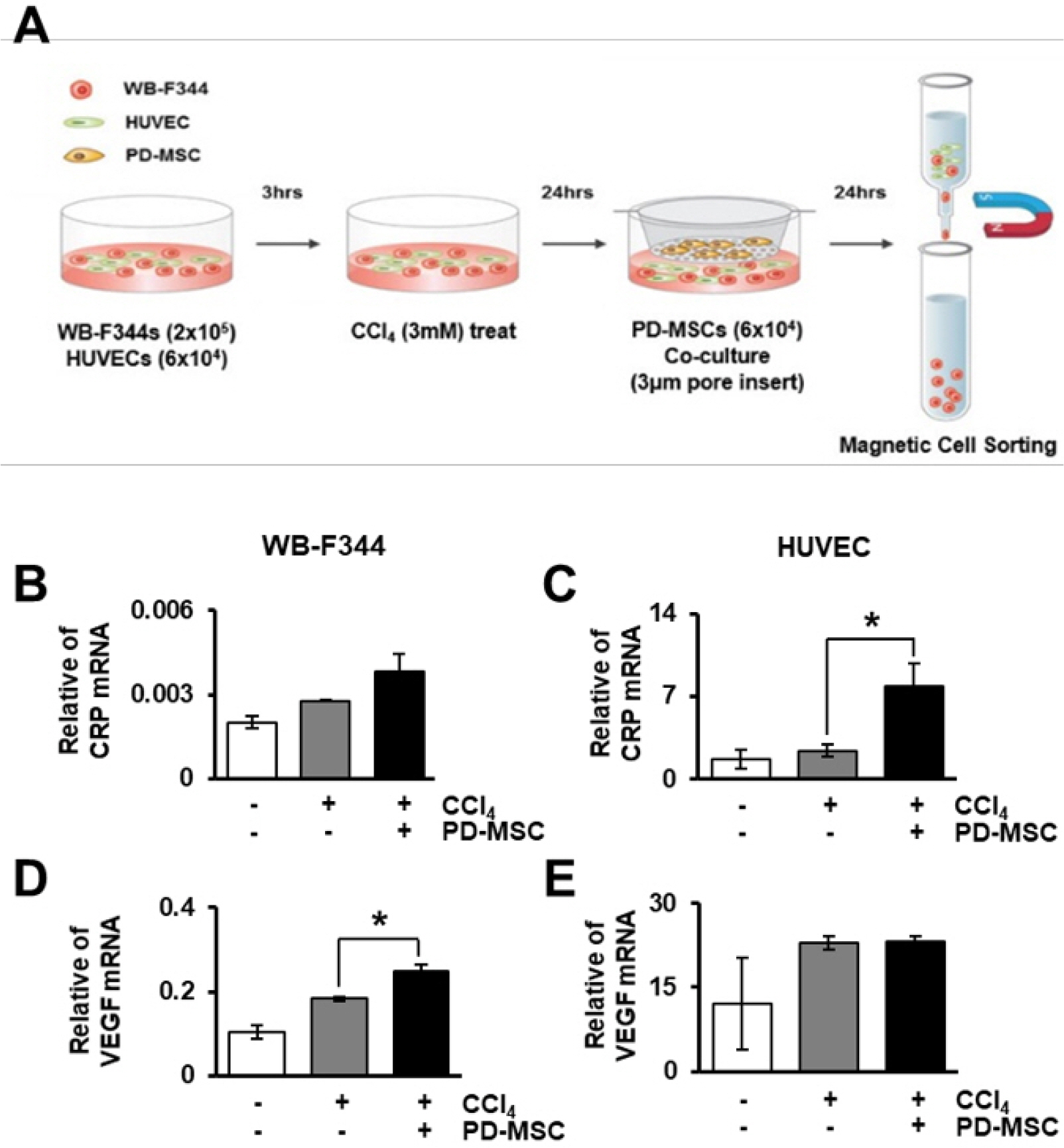
To demonstrate the interaction between hepatocytes and ECs via CRP and the Wnt signaling pathway, we used the culture scheme shown in Fig. 6A. The expression of CRP, VEGF, Axin2 and albumin was analyzed in hepatocytes, WB-F344s, and ECs, HUVECs. In sorted hepatocytes and ECs, CRP mRNA was upregulated by co-culture with PD-MSCs (Fig. 6B and 6C). Additionally, the expression of VEGF and Axin2 was increased in CCl4-treated and PD-MSC co-cultured hepatocytes and ECs (Fig. 6D and 6E, Supplementary Fig. S3A and S3B). Furthermore, albumin, a hepatocyte marker, was upregulated in hepatocytes co-cultured with PD-MSCs (Supplementary Fig. S3C). However, albumin was not detected in the sorted ECs (Supplementary Fig. S3D). The expression of CRP protein, as detected by western blotting, was increased in the CCl4-treated and PD-MSC co-cultured rat hepatocytes (Supplementary Fig. S4A and S4B). Furthermore, the Wnt pathway (Supplementary Fig. S4A, S4C and S4E), angiogenesis (Supplementary Fig. S4A and S4F) and hepatic regeneration (Supplementary Fig. S4A and S4D) were activated in the co-cultured group. Comparing between only hepatocytes culture and EC cells and hepatocytes on co-culture systems, there was no difference between liver regeneration by expression of albumin. However, the difference between vascular regeneration and Wnt signaling factors was more dynamic on co-culture systems of EC cells and hepatocytes because the VEGF secretion is exceptionally shown in the EC rather than the WB-F344. Therefore, we finally co-cultivated EC and hepatocytes to study hepatic regeneration by vascular regeneration and demonstrated CRP increased by PD-MSCs co-cultivation prompts vascular regeneration through Wnt signaling in hepatocytes by interacting with ECs.
Hepatic cirrhosis is characterized by changes in nodule and hepatic sinusoidal re-capillarization (19). In addition to hepatic stellate cells, other non-parenchymal cells such as ECs are the major co-modulators of liver fibrosis (20). Angiogenic process by ECs is complicated and dynamic under physiological and pathological conditions (21). Recent studies have demonstrated that angiogenesis and sinusoidal remodeling are extensively correlated with hepatic fibrosis and regeneration (22). Therefore, we investigated the interaction of hepatocytes with ECs, through a direct co-culture system to determine the effects of this interaction on angiogenesis.
Mice with β-catenin knockout in the liver (β-ca-tenin-LKO) revealed a significant role of β-catenin related to metabolic zonation, where β-catenin controls liver regeneration after hepatectomy by inducing cyclin-D1 (23). Similarly, Yang and colleagues made liver-specific Lrp5/6 knockout (Lrp-LKO) mice and showed that the Wnt pathway was limited in hepatocytes in the knockout model. Similar that in β-catenin-LKO mice, in Lrp-LKO mice, metabolic zonation was exacerbated by the lack of Cyp1a2 and Cyp2e1. Additionally, Lrp-LKO resulted in a signi-ficant delay in liver regeneration owing to the lack of β-catenin-TCF association and cyclin-D1 (24). Therefore, Wnt signaling in hepatocytes was identified as an important regulator of metabolic zonation and hepatic deve-lopment. In another study, Leibing et al. (25) used endothelial subtype-specific Stab2-Cre driver mice to eliminate Wnt ligands from liver ECs to investigate the effects of the liver angiocrine Wnt pathway on liver growth and metabolic functions. The suppression of the angiocrine Wnt signaling pathway exacerbated metabolic zonation in the liver through the loss of β-catenin-dependent genes such as Axin2, glutamine synthase, and cytochrome P450 2E1. Similarly, through BrdU incorporation, we confirmed that increased endothelial markers and β-catenin can promote the proliferation of rat hepatocytes (Fig. 5).
Hepatic progenitor cells (HPCs) are a subtype of liver cancer stem cells that activate the Wnt/β-catenin pathway and Notch signaling involved in angiogenesis. Notch and Hedgehog signaling pathways serve as powerful cancer-prevention mechanisms. Oishi et al. (26) demonstrated that β-catenin is upregulated in HPCs, which promotes vasculogenesis and maintains the stem cell niche. We found that upregulation of β-catenin and inhibition of pGSK3 by PD-MSCs activate vascularization through upregulating VEGF and VEGFR2 in vivo and in vitro (Fig. 3, 6, Supplementary Fig. S1, S4).
Our results indicated that the CRP is increased in the hepatic failure model in response to PD-MSC transplan-tation, correlating with IL-10 and anti-inflammatory factor expression. However, CRP has been established as an acute inflammatory marker because it has pattern-recognition activity. Although the serum level of CRP is associated with the risk and prognosis of several types of diseases, it is uncertain whether CRP is directly involved in disease or whether it is simply a bystander (27). In this study, we demonstrated a correlation between CRP and Wnt signaling in CCl4-treated rat livers transplanted PD-MSCs and rat hepatocytes co-cultured with PD-MSCs (Fig. 3D, Supplementary Fig. S4). Similar with our study, Su and colleagues identified mutations at SNP-286 (rs3091244) in the CRP promoter. Further analysis showed that the CRP mutations at SNP-286 co-occur with mutated adenomatous polyposis coli, which functions in the Wnt/β-catenin pathway (28).
Pena and colleagues investigated the effects of CRP on angiogenic function in vascular ECs. mCRP activates the TF pathway in mouse ECs by phosphorylating AKT and promoting the expression of the transcription factor ETS1, leading to increased CCL2. Additionally, circulating pCRP, with mild pro-angiogenic effect via dissociation into mCRP on the surface of mouse ECs, can trigger potent angiogenic effects by enhancing the upregulation of the F3 gene and TF signaling (9). Chen et al. (29) confirmed that CRP affects the proliferation and pro-angiogenic paracrine activity of adipose-derived stem cells (ADSCs). In their study, CRP did not affect ADSC proliferation or apoptosis, but it induced ADSC migration by promoting the PI3K/AKT pathway. ADSCs treated CRP upregulated VEGF-A by inducing hypoxia inducible factor-1α, which enhanced tube formation on matrigel. Similarly, we found that CRP induction by PD-MSCs enhanced vascularization in CCl4-injured rat livers and hepatocytes co-cultured with ECs (Fig. 3, 6, Supplementary Fig. S4).
In conclusion, PD-MSCs increase the expression of CRP, which attenuates inflammation. The upregulated CRP by PD-MSCs activates Wnt signaling and angiogenesis and facilitates hepatocyte regeneration in vitro and in vivo. These results indicate that CRP induced by PD-MSCs is involved in vascular remodeling via the Wnt signaling during liver regeneration after hepatic failure and support the role of CRP as a hepatic regenerative factor.
Supplementary data including four figures can be found with this article online at http://pdf.medrang.co.kr/paper/pdf/IJSC/IJSC-13-s20052.pdf.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI17C1050) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2020M3A9B302618221).
Notes
Author Contributions
J.H.J.: study concept and design, data acquisition, and drafting of the manuscript; J.J. and J.Y.K: data analysis; S.G.H. and S.H.B.: data interpretation, critical discussion; G.J.K.: conceived and designed the experiments and directed manuscript drafting, financial support and final approval of manuscript.
References
1. Rui L. 2014; Energy metabolism in the liver. Compr Physiol. 4:177–197. DOI: 10.1002/cphy.c130024. PMID: 24692138. PMCID: PMC4050641.

2. Poisson J, Lemoinne S, Boulanger C, Durand F, Moreau R, Valla D, Rautou PE. 2017; Liver sinusoidal endothelial cells: physiology and role in liver diseases. J Hepatol. 66:212–227. DOI: 10.1016/j.jhep.2016.07.009. PMID: 27423426.

3. Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D, Sato TN, Rabbany SY, Rafii S. 2010; Inductive angiocrine signals from sinusoidal endothelium are required for liver regene-ration. Nature. 468:310–315. DOI: 10.1038/nature09493. PMID: 21068842. PMCID: PMC3058628.

4. Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S. 2014; Divergent angiocrine signals from vascular niche balance liver regene-ration and fibrosis. Nature. 505:97–102. DOI: 10.1038/nature12681. PMID: 24256728. PMCID: PMC4142699.

5. Pinzani M, Rosselli M, Zuckermann M. 2011; Liver cirrhosis. Best Pract Res Clin Gastroenterol. 25:281–290. DOI: 10.1016/j.bpg.2011.02.009. PMID: 21497745.

6. Salazar J, Martínez MS, Chávez-Castillo M, Núñez V, Añez R, Torres Y, Toledo A, Chacín M, Silva C, Pacheco E, Rojas J, Bermúdez V. 2014; C-reactive protein: an in-depth look into structure, function, and regulation. Int Sch Res Notices. 2014:653045. DOI: 10.1155/2014/653045. PMID: 27433484. PMCID: PMC4897210.

7. Eisenhardt SU, Habersberger J, Murphy A, Chen YC, Woollard KJ, Bassler N, Qian H, von Zur Muhlen C, Hagemeyer CE, Ahrens I, Chin-Dusting J, Bobik A, Peter K. 2009; Dissociation of pentameric to monomeric C-reactive protein on activated platelets localizes inflammation to atherosclerotic plaques. Circ Res. 105:128–137. DOI: 10.1161/CIRCRESAHA.108.190611. PMID: 19520972.

8. Eisenhardt SU, Thiele JR, Bannasch H, Stark GB, Peter K. 2009; C-reactive protein: how conformational changes influence inflammatory properties. Cell Cycle. 8:3885–3892. DOI: 10.4161/cc.8.23.10068. PMID: 19887916.

9. Peña E, de la Torre R, Arderiu G, Slevin M, Badimon L. 2017; mCRP triggers angiogenesis by inducing F3 transcription and TF signalling in microvascular endothelial cells. Thromb Haemost. 117:357–370. DOI: 10.1160/TH16-07-0524. PMID: 27808345.

10. Krayem I, Bazzi S, Karam M. 2017; The combination of CRP isoforms with oxLDL decreases TNF-α and IL-6 release by U937-derived macrophages. Biomed Rep. 7:272–276. DOI: 10.3892/br.2017.949. PMID: 28808571. PMCID: PMC5543421.

11. Clevers H. 2006; Wnt/beta-catenin signaling in development and disease. Cell. 127:469–480. DOI: 10.1016/j.cell.2006.10.018. PMID: 17081971.
12. Thompson MD, Monga SP. 2007; WNT/beta-catenin signaling in liver health and disease. Hepatology. 45:1298–1305. DOI: 10.1002/hep.21651. PMID: 17464972.
13. Korn C, Scholz B, Hu J, Srivastava K, Wojtarowicz J, Arnsperger T, Adams RH, Boutros M, Augustin HG, Augustin I. 2014; Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis. Development. 141:1757–1766. DOI: 10.1242/dev.104422. PMID: 24715464.

14. Tran KA, Zhang X, Predescu D, Huang X, Machado RF, Göthert JR, Malik AB, Valyi-Nagy T, Zhao YY. 2016; Endothelial β-catenin signaling is required for maintaining adult blood-brain barrier integrity and central nervous system homeostasis. Circulation. 133:177–186. DOI: 10.1161/CIRCULATIONAHA.115.015982. PMID: 26538583. PMCID: PMC4814374.

15. Lee HJ, Jung J, Cho KJ, Lee CK, Hwang SG, Kim GJ. 2012; Comparison of in vitro hepatogenic differentiation potential between various placenta-derived stem cells and other adult stem cells as an alternative source of functional hepatocytes. Differentiation. 84:223–231. DOI: 10.1016/j.diff.2012.05.007. PMID: 22885322.

16. Zhang D, Jiang M, Miao D. 2011; Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse. PLoS One. 6:e16789. DOI: 10.1371/journal.pone.0016789. PMID: 21326862. PMCID: PMC3033905.

17. Jung J, Moon JW, Choi JH, Lee YW, Park SH, Kim GJ. 2015; Epigenetic alterations of IL-6/STAT3 signaling by placental stem cells promote hepatic regeneration in a rat model with CCl4-induced liver injury. Int J Stem Cells. 8:79–89. DOI: 10.15283/ijsc.2015.8.1.79. PMID: 26019757. PMCID: PMC4445712.

18. Jun JH, Choi JH, Bae SH, Oh SH, Kim GJ. 2016; Decreased C-reactive protein induces abnormal vascular structure in a rat model of liver dysfunction induced by bile duct ligation. Clin Mol Hepatol. 22:372–381. DOI: 10.3350/cmh.2016.0032. PMID: 27729629. PMCID: PMC5066379.

19. Xu B, Broome U, Uzunel M, Nava S, Ge X, Kumagai-Braesch M, Hultenby K, Christensson B, Ericzon BG, Holgersson J, Sumitran-Holgersson S. 2003; Capillarization of hepatic sinusoid by liver endothelial cell-reactive autoantibodies in patients with cirrhosis and chronic hepatitis. Am J Pathol. 163:1275–1289. DOI: 10.1016/S0002-9440(10)63487-6. PMID: 14507637. PMCID: PMC1868294.

20. Iredale JP. 2007; Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 117:539–548. DOI: 10.1172/JCI30542. PMID: 17332881. PMCID: PMC1804370.

21. Djonov V, Baum O, Burri PH. 2003; Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 314:107–117. DOI: 10.1007/s00441-003-0784-3. PMID: 14574551.

22. Park S, Kim JW, Kim JH, Lim CW, Kim B. 2015; Differential roles of angiogenesis in the induction of fibrogenesis and the resolution of fibrosis in liver. Biol Pharm Bull. 38:980–985. DOI: 10.1248/bpb.b15-00325. PMID: 26133707.

23. Yang J, Mowry LE, Nejak-Bowen KN, Okabe H, Diegel CR, Lang RA, Williams BO, Monga SP. 2014; β-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation! Hepatology. 60:964–976. DOI: 10.1002/hep.27082. PMID: 24700412. PMCID: PMC4139486.

24. Preziosi M, Okabe H, Poddar M, Singh S, Monga SP. 2018; Endothelial Wnts regulate β-catenin signaling in murine liver zonation and regeneration: a sequel to the Wnt-Wnt situation. Hepatol Commun. 2:845–860. DOI: 10.1002/hep4.1196. PMID: 30027142. PMCID: PMC6049069.

25. Leibing T, Géraud C, Augustin I, Boutros M, Augustin HG, Okun JG, Langhans CD, Zierow J, Wohlfeil SA, Olsavszky V, Schledzewski K, Goerdt S, Koch PS. 2018; Angiocrine Wnt signaling controls liver growth and metabolic maturation in mice. Hepatology. 68:707–722. DOI: 10.1002/hep.29613. PMID: 29059455. PMCID: PMC6099291.

26. Oishi N, Yamashita T, Kaneko S. 2014; Molecular biology of liver cancer stem cells. Liver Cancer. 3:71–84. DOI: 10.1159/000343863. PMID: 24944998. PMCID: PMC4057789.

27. Moutachakkir M, Lamrani Hanchi A, Baraou A, Boukhira A, Chellak S. 2017; Immunoanalytical characteristics of C-reactive protein and high sensitivity C-reactive protein. Ann Biol Clin (Paris). 75:225–229. DOI: 10.1684/abc.2017.1232. PMID: 28377336.

28. Su HX, Zhou HH, Wang MY, Cheng J, Zhang SC, Hui F, Chen XZ, Liu SH, Liu QJ, Zhu ZJ, Hu QR, Wu Y, Ji SR. 2014; Mutations of C-reactive protein (CRP) -286 SNP, APC and p53 in colorectal cancer: implication for a CRP-Wnt crosstalk. PLoS One. 9:e102418. DOI: 10.1371/journal.pone.0102418. PMID: 25025473. PMCID: PMC4099363.

29. Chen J, Gu Z, Wu M, Yang Y, Zhang J, Ou J, Zuo Z, Wang J, Chen Y. 2016; C-reactive protein can upregulate VEGF expression to promote ADSC-induced angiogenesis by activating HIF-1α via CD64/PI3k/Akt and MAPK/ERK signaling pathways. Stem Cell Res Ther. 7:114. DOI: 10.1186/s13287-016-0377-1. PMID: 27526687. PMCID: PMC4986362.
Fig. 1
PD-MSC transplantation attenuates inflammation in CCl4-injured rats. Expression of NF-κB was detected by immunohistochemistry (A). ×400, Scale bars; 20 μm. NF-κB-positive cells were quantified based on the total number of cells (B). Expression of IL-10 (C) and IL-6 (D) was analyzed in rat serum by ELISA. Data are represented as the triplicated mean±SD. p<0.05, *; NTX vs.; NTX and TTX refer to the non-transplanted group and the PD-MSC-transplanted group, respectively.
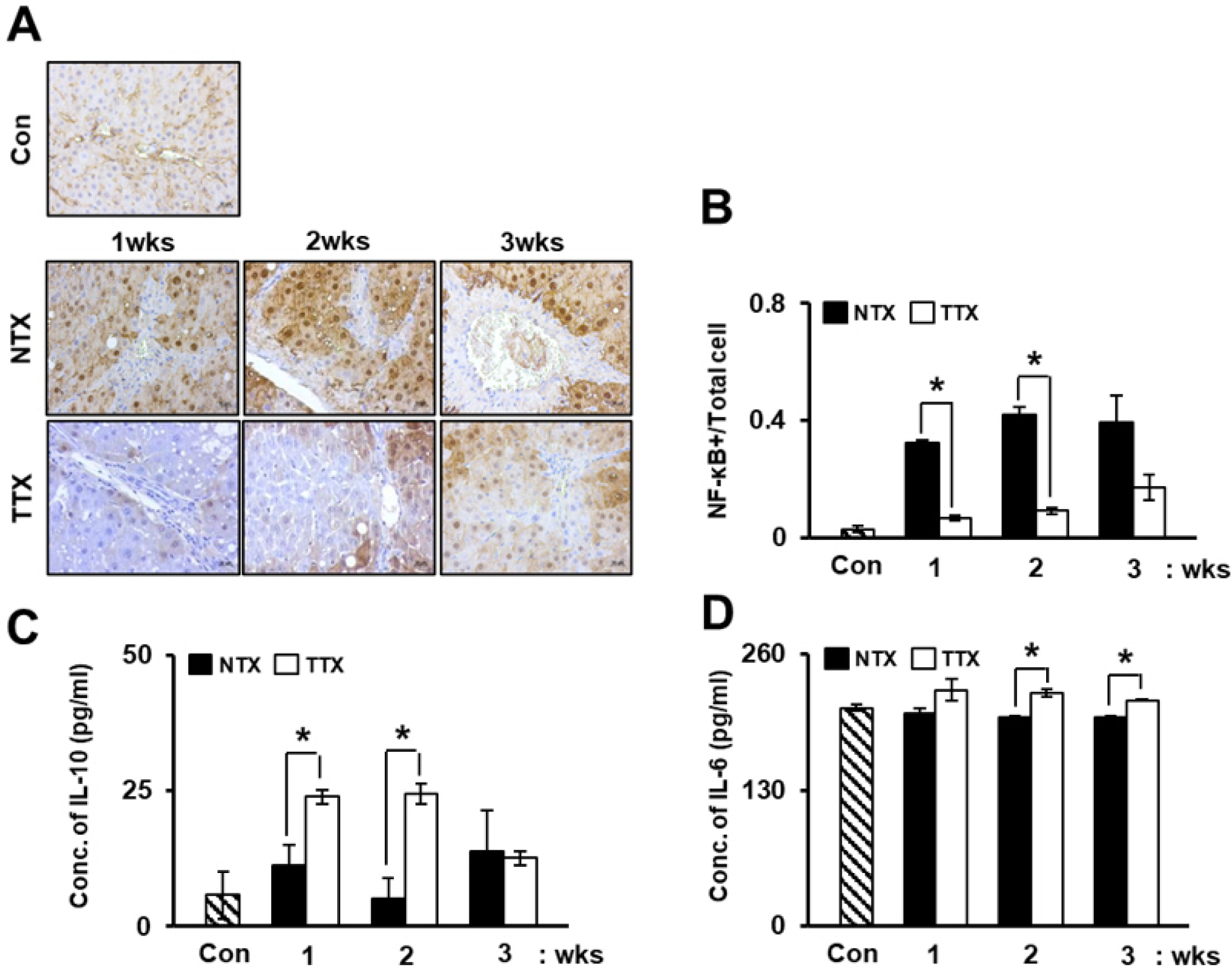
Fig. 2
PD-MSC transplantation improves the expression of CRP. Expre-ssion of CRP was analyzed by ELISA (A) and Western blotting from total proteins (C) and exosomes (B) and (D). Data are represented as the triplicated or duplicated mean±SD. SD; p<0.05, *; NTX vs.; NTX and TTX refer to the non-transplanted group and the PD-MSC-transplanted group, respectively.
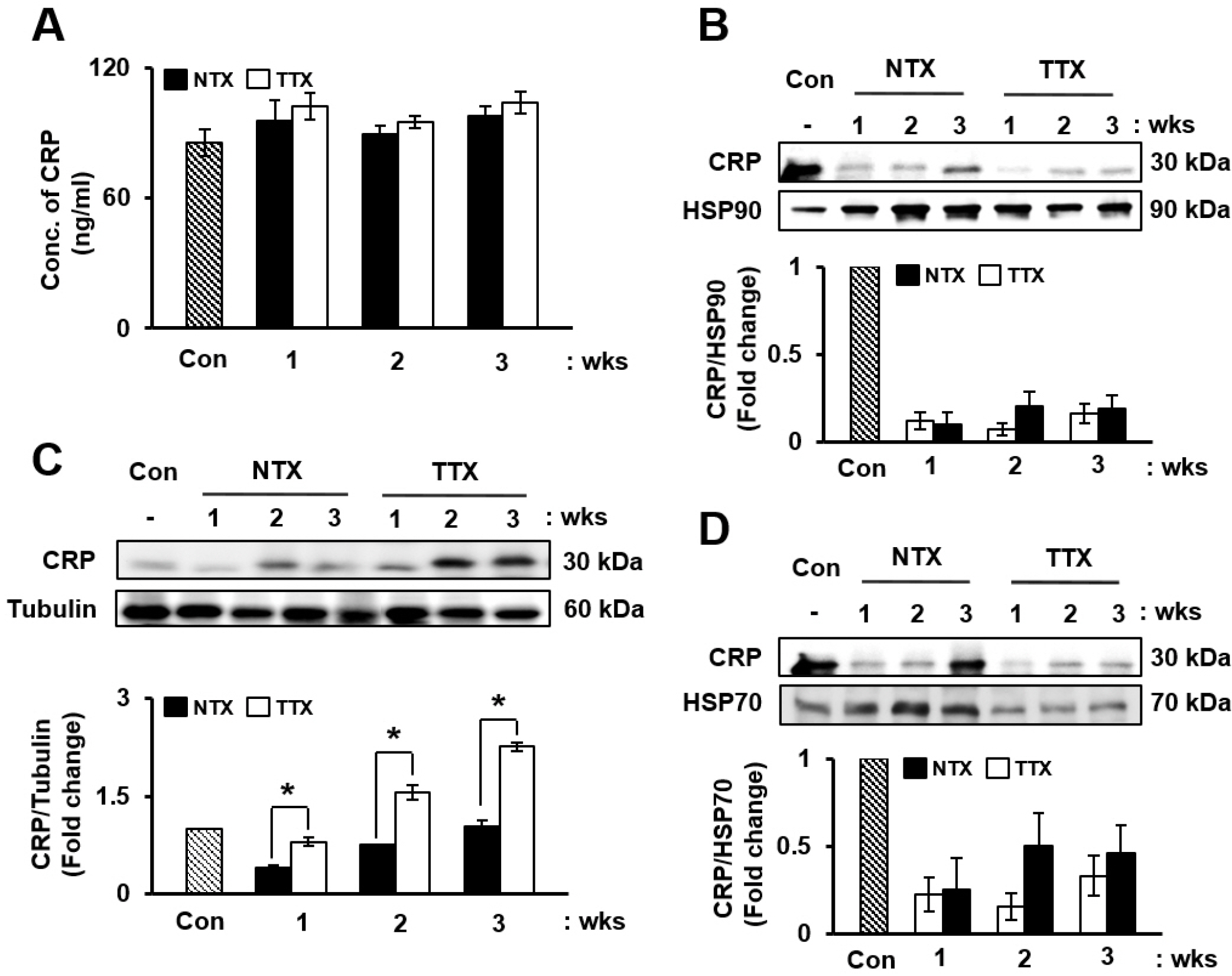
Fig. 3
PD-MSC transplantation induces Wnt signaling and angiogene-sis in CCl4-injured rats. Angiogenic (A) and Wnt signaling (B) factors were detected by Western blotting. Localization and expression of VEGF and β-catenin visualized using immunofluorescence (C). ×630, Scale bars; 20 μm. Positive correlations were found between CRP and β-catenin (D) also, between β-catenin and VEGF (E). NTX and TTX refer to the non-transplanted group and the PD-MSC-transplanted group, respec-tively.
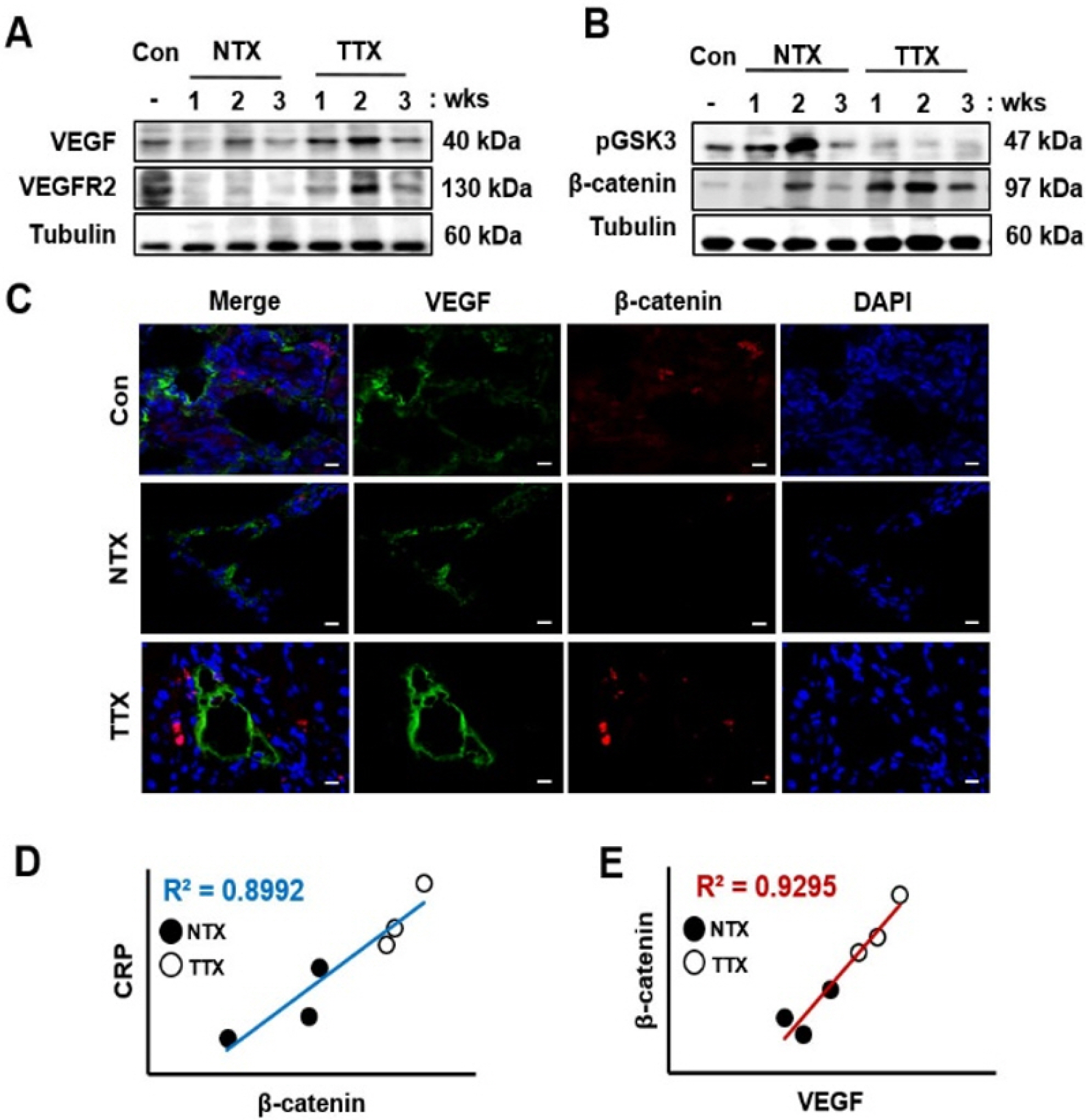
Fig. 4
CRP regulates angiogenesis and Wnt signaling in rat hepatocytes (WB-F344s). Protein levels of CRP (A), VEGF (B), β-catenin (C), ALB (D), HNF1α (E), and CyclinD1 (F) were evaluated in siRNA-CRP-transfected rat hepatocytes by western blot. Data are represented as the triplicated mean±SD of. *, p<0.05. Mock and si-CRP refer to non-transfected and siRNA-CRP-transfected rat hepatocytes, respectively.
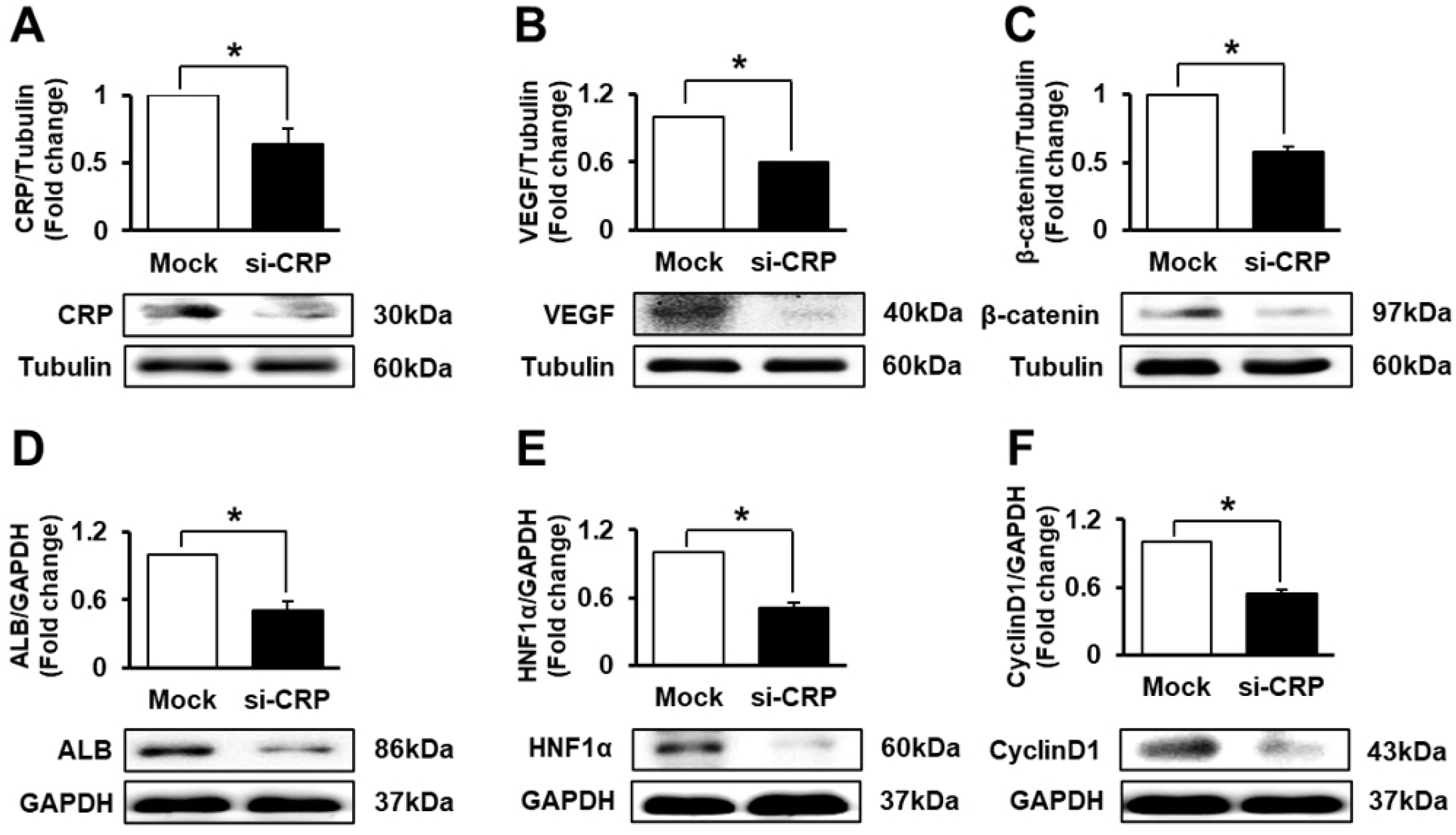
Fig. 5
PD-MSCs promote the expression of CRP and proliferation of hepatocytes through Wnt signaling. Expression of CRP was analyzed by qRT-PCR (A) and ELISA (B) after CCl4 treatment and co-culture with PD-MSCs. Immunofluorescence staining shows the localization and expression of BrdU and β-catenin in rat hepatocytes treated with CCl4 and co-cultured with PD-MSCs (C). ×400, Scale bars; 20 μm. BrdU- or β-ca-tenin-(D) positive rat hepatocytes were quantified after immunofluore-scence staining. Protein levels of HNF1α, and CyclinD1 were detected by western blotting in rat hepatocytes treated with CCl4 and co-cultured with PD-MSCs (E). Intensity of HNF1α (F) and Cyclin D1 (G) protein bands was calculated. Tripli-cated data are represented as the mean±SD. *, p<0.05.
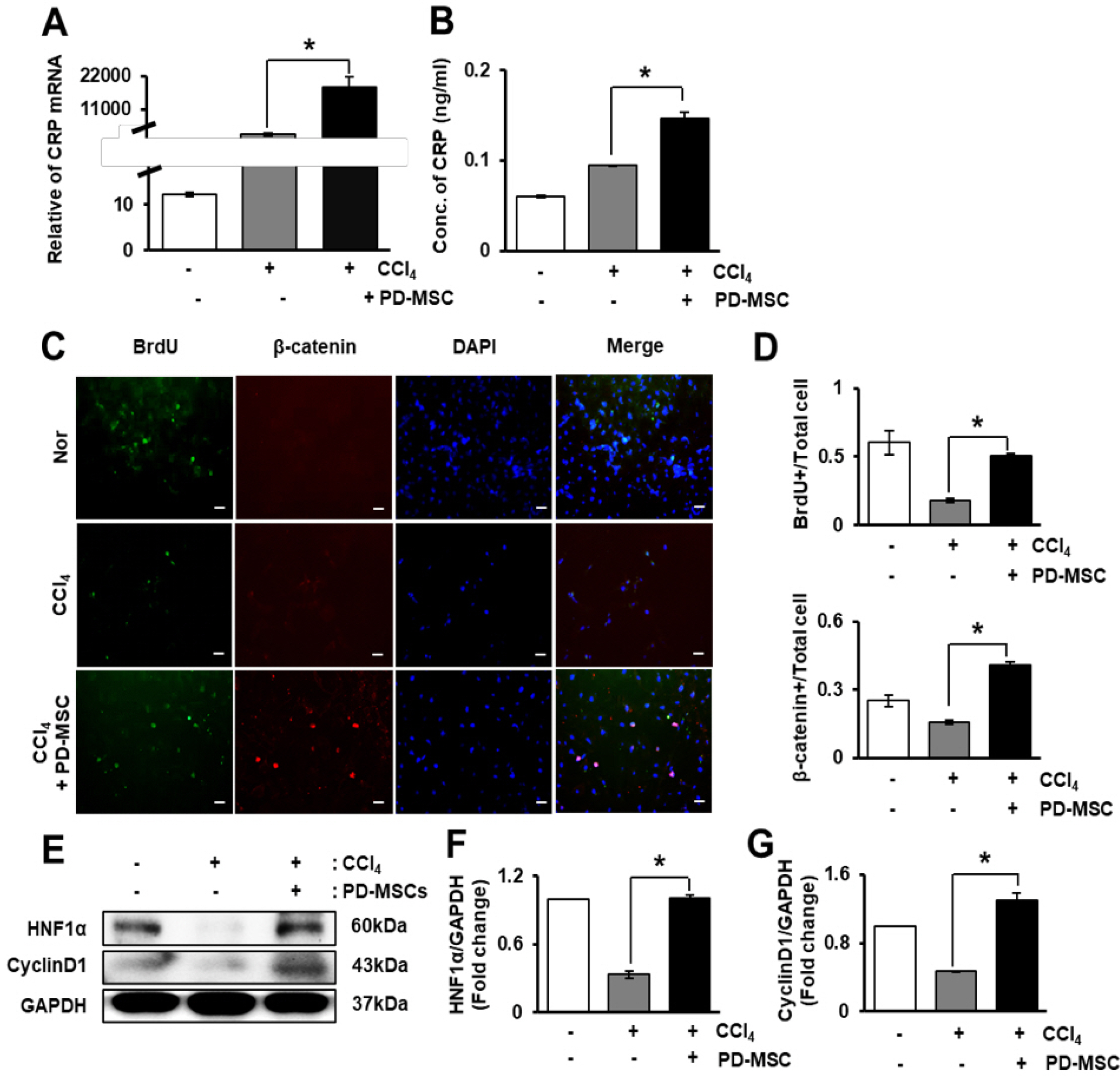
Fig. 6
PD-MSCs induce the expression of CRP and VEGF at the mRNA level. Schematic showing the experimental protocol (A). mRNA level of CRP (B), VEGF (D) was quantified in rat hepatocytes treated with CCl4 and co-cultured with HUVECs and PD-MSCs. mRNA level of CRP (C), VEGF (E) was assayed in HUVECs after CCl4 treatment and co-culture with rat hepatocytes and PD-MSCs. Duplicated data are represented as the mean±SD. *, p<0.05.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download