Abstract
Recently, evidences show that cancer stem cells (CSCs) are a type of cancer cell group with self-renewal and play a huge role in tumor recurrence, metastasis, and drug resistance. Finding new treatment directions and targets for cancer prognosis and reducing mortality has become a top priority. OCT4, as a transcription factor, participates in maintaining the stem characteristics of CSCs, but the mechanism of OCT4 is often overlooked. In this review, we try to illustrate the mechanism by which OCT4 plays a role in CSCs from the perspective of genetic modification of OCT4, non-coding RNA, complexes and signaling pathways associated with OCT4. Our ultimate goal is to provide new targets for cancer treatment to prolong the survival of cancer patients.
Cancer stem cells (CSCs) are a type of cell population with self-renewal and replication that have been found to be the origin of cancer (1, 2). Growing evidence indicates that CSCs are tumor-initiating cells and play a non-negligible role in cancer recurrence, metastasis, and drug resistance (1, 3, 4). However, it is unclear how CSCs are generated and maintain stemness, which make tumor treatment challenging.
Octamer-binding transcription factor 4 (OCT4), which is encoded by the Pou5f1 gene and is a member of the POU-domain transcription factor family, is connected to maintain pluripotency of embryonic stem cells (ESCs) and CSCs (5-7). Mouse Oct4 has two homologs, Oct4A and Oct4B, the former in the nucleus and the latter in the cytoplasm (8). Unlike mice, human OCT4 has three homologs, OCT4A, OCT4B and OCT4B1 (9). One study found that Oct4 expression was not detectable in adult murine organs (10). Meanwhile, some researchers also suggested that although Oct4 expression cannot be detected in testes, brain, liver, lung, kidney, and intestine, there is Oct4 expression in primordial germ cells and unfertilized oocytes, which shows that differential regulation of Oct4 expre-ssion during mouse development (11). However, a study has found that there is Oct4 mRNA expression in the murine adult ovaries and testes, especially mature and ovulating oocytes rather than resting oocytes, which may be due to Oct4 transactivate genes that are important for oocyte maturation (12). Gradually, some researchers suggest that Oct4 is necessary and sufficient to induce pluripotency of adult mouse neural stem cells (13). The expression of OCT4 in several human adult stem cells, such as breast, pancreatic, and liver stem cells, supports the hypothesis that stem cells are carcinogenic target cells (14). Additionally, OCT4 may generate resistance to radiotherapy by improving the epithelial to mesenchymal transition (EMT) process in human rectal cancer cells, and OCT4 is closely related to DNA damage when cancer cells respond to radiotherapy (15). Studies also indicate that OCT4 is strongly associated with tumor invasion and migration and can lead to poor prognosis for patients (6). Unfortunately, studies on the functions and mechanisms of OCT4 in the production of CSCs are scarce. The purpose of this review is to summarize the relationship between OCT4 and CSCs to provide possibilities for clinical treatment by exploring targets related to OCT4.
The POU domain, which name derived from the Pituary-specific TF Pit1, Octamer binding TFs Oct1 and Oct2, and neural TF Unc-86, is highly conserved DNA-banding domain (16, 17). OCT4 has two DNA-binding domains, which bind to octamers of free DNA (octamer motif 5’-ATTTGCAT-3’) in a sequence-specific manner and induce chromatin opening and regulate gene expression (17, 18). The two DNA-binding domains consist of a POU-specific domain (POUS) and a POU homeodomain (POUHD) (19). Compared with other stem genes, OCT4 has universal expression in CSCs of hepatocellular carcinoma, breast cancer, prostate cancer, melanoma, osteosarcoma, bladder cancer, ovarian cancer, and lung cancer (7, 20, 21). Oct4 is overexpressed in pluripotent embryonic cells and silenced after cell differentiation during mouse embryonic development (9). It seems that OCT4 can be a pluripotency and germ cell marker and be used to distinguish from non-CSCs, and participate in determining the biological function of CSCs.
Epigenetic modification is a reversible and heritable change in gene function when the DNA sequence has not changed, which can participate in important biological processes by regulating gene expression (22). OCT4 silencing plays important role in differentiation, cell engineering, and tumors (23). In contrast, OCT4 expression is related to stem cell characteristics and increases spheroid formation capacity (24).
Studies have shown that OCT4 is regulated by DNA methylation of CpG of the promoter and exon in the human trophoblast cells and ESCs (25, 26). Hypermethy-lation of the Oct4 promoter and enhancer regions results in structural changes in chromatin and inhibits Oct4 expression in mouse trophoblast stem cells (27). However, the epigenetic regulation of the histones in the OCT4 promoter region cannot be ignored. The OCT4 promoter region includes the CCCTC-binding factor (CTCF) binding site. As it happens, Brother of the Regulator of Imprinted Sites (BORIS) as a CTCF paralog can bind to the OCT4 promoter, and it promotes OCT4 expression and promotes stemness of human hepatocellular carcinoma by up-regulating H3K4me2 and down-regulating the level of H3K27me3 (28).
Researchers indicated that acetylation of OCT4 and SOX2 can attenuate transcriptional activity by impairing OCT4/SOX2 heterodimer formation (29). The expression of OCT4 is positively correlated with Ca2+/calmodulin-dependent protein kinase IIγ (CaMKIIγ), which promotes the acetylation of the histones of OCT4 by activating Akt, thereby maintaining the stem cell capacity and tumorigenicity of human lung cancer cells (30). H3K56 acetylation is highly conserved in organisms, which can interact with Oct4 and promote the pluripotency of mouse ESCs (mESCs) (31). A significant increase in the acetylation level of H3K9 in the OCT4 promoter region was found in spherical cultures formed from human mesenchymal stem cells (32), which shows that the post-translational modification of OCT4’s histones plays an important role in stem maintenance.
Phosphorylation that occurs in the POUHD region of OCT4 in human ESCs (hESCs) inhibits its activity by blocking sequence-specific DNA binding (19). Studies have also found that Akt-mediated OCT4 phosphorylation can regulate stemness by changing the interaction with SOX2 (29). And increasing evidence suggests that phosphorylation at threonine 343 of Oct4 is also critical for maintaining mESC pluripotency (33). In addition, different genetic modifications also interact with each other. The phosphorylation of human OCT4 at serine 111 promotes its ubiquitination, which affects its activity and cell localization (34).
Experiments show that E3 ubiquitin ligase WWP2 promotes the ubiquitination and degradation of OCT4 in hESCs. The same result is also observed in differentiated mouse embryonic carcinoma cells. Furthermore, ITCH, another ligase, regulates mESC Oct4 transcription and degradation after ubiquitination (9, 34, 35). Carboxy terminus of HSP70-interacting protein (CHIP), which is an E3 ubiquitin ligase, can ubiquitinate OCT4 at lysine 284, which can reduce OCT4 stability and subsequently inhibit human breast CSC production (36). Oct4 SUMOylation in mouse embryonic carcinoma cells results in enhanced protein stability, transactivation function and DNA binding (7, 29). Studies have found that OCT4 expression increases in cells of rectal cancer, neuroblastoma, and melanoma after drug treatment. The difference is that testicular germ cell tumors treated with cisplatin appear to reduce OCT4 expression through SUMOylation, but specific mechanism is still unclear (37). There is evidence that the protein activity of human OCT4 is altered by monosaccharide O-linked β-N-acetylglucosamine (O-GlcN Ac) (9). O-GlcNAc at the threonine 228 of Oct4 boosts the transcription of Oct4 and thus induces a variety of pluripotency genes, which is essential for the reprogramming of mESCs (38).
In summary, the same genetic modification may regulate the structure and function of OCT4 through different pathways due to the role of different enzymes. Various genetic modifications participate in the regulation of OCT4 activity, stability and cell localization through interaction in various forms (Fig. 1), all of which have an effect on the role of OCT4 and provide new perspectives and targets for cancer treatment.
There are many types of non-coding RNA (ncRNA), such as long non-coding RNA (lncRNA), small interfering RNA (siRNA) and micro RNA (miRNA, miR) (39). Non-coding RNA is involved in cell growth, differentia-tion, apoptosis, invasion, and other important biological processes, and even some of them have proven to be crucial in the development of cancer (40, 41). OCT4, as a representative CSC marker, is often used to judge the role of ncRNA in CSCs, such as miR-30b, lncRNA HOXA11-AS, and lncRNA MEG3 (42-44). In addition, more and more ncRNAs have been shown to be related to the regulation of CSCs by interacting with OCT4.
OCT4 binds to the lncRNA NETA1.1 promoter in human bladder cancer-resistant cells treated with cisplatin, making it highly expressed to maintain the invasion and growth of bladder cancer cells (45). LncRNA CCAT2 overexpressed in human breast CSCs maintains the aggressiveness of CSCs by up-regulating the OCT4 pseudogene (OCT4-PG1) (46). The ability of MALAT1 to maintain the stemness of human colon CSCs may be achieved by targeted inhibition of miR-20b-5p and reducing the binding of miR-20b-5p to OCT4 mRNA (Pou5f1) (47). OCT4 promotes the invasion and proliferation of cancer cells by regulating the level of lncRNA AK055347 in human osteosarcoma cells (48). The activation of lncRNA AK028326 was also found to be directly regulated by Oct4 in mESCs (9). Lnc-CRCMSL, as an anti-metastatic gene, prevents human colorectal cancer cells from reprogramming by inhibiting high mobility group box 2 (HMGB2) from metastasizing to the nucleus and inhibiting the interaction between HMGB2 and OCT4 (49). H19 can directly regulate the expression of OCT4 in human prostate cells. In turn, in F9 embryonic carcinoma cells, Oct4 and Sox2 can also positively regulate H19 by weakening the methylation level of the imprinted control region and promoter (50, 51). And research has also found that lncRNA ROR can form a regulatory feedback loop with OCT4, SOX2, and NANOG in hESCs (52, 53).
Further evidence suggests that the involvement of miRNA in maintaining pluripotency is due to the presence of OCT4 binding sites on miRNA promoters (54). It is worth noting that OCT4, SOX2 and NANOG can bind to the promoter of miR-302 and promote its expression in hESCs (55). The miR-302 can also indirectly positively regulate OCT4 activity to promote reprogramming efficiencies from human adipose-derived stem cells into induced pluripotent stem cells through targeted inhibition of Nuclear receptor subfamily 2, group F, member 2 (NR2F2) that suppresses OCT4 promoter activity (56). MiR-145 directly targets the 3’UTR of OCT4, which inhibits the transcription of OCT4 and promotes the differentiation and inhibits the proliferation of human endometrial adenocarcinoma cells (57), and the same result was also found in glioblastoma (58). Coincidentally, OCT4 also binds to the promoter of miR-145 to achieve functional inhibition of miR-145, which seems to indicate that a double-negative feedback loop can be formed between miR-145 and OCT4 in hESCs (9, 59). The lncRNA linc-DYNC2H1-4, which present in the cytoplasm of gemcitabine-resistant pancreatic cancer cells, competes with miR-145 and subsequently eliminates the targeted inhibi-tion of OCT4 by miR-145 to promotes CSC phenotypes (60). Studies in endometrial cancer and hepatocellular carcinoma have still found that the targeted inhibition of OCT4 by miR-145 can be reversed by OCT4 pseudogene 5 (OCT4-pg5) due to their similar binding sites in OCT4 3’UTR (61, 62). In addition, a decrease of lincRNA ROR in prostate CSCs can increase the effective concentration of miR-145 and inhibit CSC proliferation (50, 63). In short, many ncRNAs may use miR-145 as an intermediate to complete the connection with OCT4. MiR-302/367 cluster regulated by OCT4 plays a role in maintaining pluripotency of stem cells (54). OCT4 directly regulates miR-1246, and they collectively activate the Wnt/β-catenin signaling pathway, which can promote self-renewal, tumorigenicity and drug resistance of liver CSCs (64).
Using siRNA to target OCT4 may become a means to eradicate CSCs, which can achieve the purpose by inducing CSCs to age and apoptosis (65). The discovery of siOCT4 in head and neck squamous cell carcinoma CSCs inhibits EMT and resistance by targeting the increased OCT4 in CSCs, which proves a new possibility for eradicating cancer cells and reducing metastasis and recurrence (66). The same siRNA-mediated study of OCT4 targeted silencing has also been found in pancreatic cancer, showing inhibition of pancreatic cancer cell proliferation and induction of apoptosis (67). The use of OCT4 siRNA in breast CSCs reduces drug resistance to paclitaxel and tumor initiating ability (68). However, the efficiency of siRNA delivery is limited and needs to be improved. But the targeted therapy of OCT4 still provides us with new ideas for CSC treatment, which may need us to study more comprehensive technologies.
In fact, many ncRNAs can affect the expression of OCT4 when regulating CSCs, but the ncRNAs that actually interact with OCT4 are somewhat scarce. And the interaction between ncRNA and OCT4 is extremely complicated (Table 1). The ncRNAs can directly bind to OCT4, or form a regulatory network with OCT4 through an “intermediate”, which provides multiple therapeutic targets for eradicating CSCs.
Protein complexes are complexes formed by two or more functionally related proteins through disulfide bonds or other interactions, which have a huge influence in the occurrence and progression of cancer, such as cell localization, gene transcription, DNA damage repair, cell cycle, cell differentiation, and other biological processes (69, 70). OCT4 forms protein complexes with different partners (SOX2, NANOG, KLF4 or other proteins) to participate in the regulation of proliferation and self-renew of CSCs (29, 71).
Of these, the OCT4/SOX2 protein complex is common (72). The Oct4/Sox2 complex single-molecule imaging model indicates that Sox2 may first bind to the chromosome, which provides a target for subsequent Oct4 binding, and that Oct4 binding can stabilize the Oct4/Sox2 structure in mESCs (73). The OCT4/SOX2 complex induces transcription in the nucleus and leads to the concept that binding partners can stimulate nuclear localization (8). And they bind to specific target genes to induce their expression and jointly maintain CSC-like characteristics. However, whether the OCT4/SOX2 complex works may be related to post-translational modification and the specific mechanism is not clear (5, 7, 9). Subsequently, it was discovered that OCT4/Lys-156 has different post-translational modifications in human pluripotent stem cells and differentiated cells, which may affect the stability of the OCT4/SOX2 complex and regulate the EMT phenotype (73). In the structure of the OCT4/SOX2 complex, the salt bridge formed between OCT4/Lys-151 and SOX2/Asp-107 is relatively obvious, so post-translational modification or mutation of key residues may destroy the salt bridge structure and damage the stability of the protein complex, which seems to reduce the maintenance of stem cell-like characteristics by mechanically damaging the complex structure (73). The OCT4/SOX2 complex has also been shown to be involved in DNA repair, cell cycle, and apoptosis (74). Zfp206 as a transcrip-tion factor involved in maintaining pluripotent stem cells, has been shown to interact with the Oct4/Sox2 complex in mESCs and is an important part of the complex (9, 75). In head and neck squamous cell carcinoma, OCT4/SOX2/NANOG may form complexes or regulatory networks to prevent differentiation and lead to poor prognosis and chemoresistance (71).
β-catenin forms complexes with Oct4 and Klf4 and participates in regulating stem cell-like characteristics in mESCs (55). The sal-like 4 (SALL4) as a transcription factor maintains the proliferation, chemoresistance and self-renewal ability of CSCs and can form a complex or regulatory network with OCT4 to maintain CSC-like characteristics (76-78). Ku80 encoded by the XRCC5 gene is related to the repair of double-stranded DNA breaks and can interact with SALL4, thereby interfering with the stability of the SALL4/OCT4 complex and destroying the self-renewal ability of liver CSCs (76). The scaffolding protein caveolin-1 (Cav-1), which has a tumor suppressive effect and is low-expressed in the tumorspheres of breast cancer, can form a complex with OCT4 and mediate the degradation of OCT4 through ubiquitin-proteasome (79, 80). However, NO can damage the Cav-1/OCT4 complex by promoting phosphorylation on Cav-1 tyrosine 14 and boost the stability and biological function of OCT4 and lung CSC-like phenotype (79, 81). Under hypoxic or glucose-restricted conditions, nuclear PKM2, as an isozyme of pyruvate kinase, can bind to OCT4 and collectively regulate the transcription of downstream stemness-related genes, thereby increasing cancer invasion and metastasis (82, 83). The BAF (BRG1-associated factor) complex interacts with the Oct4 to form the Oct4/BAF complex, which regulates epigenetic modifications of mESC differentia-tion (84). Functional proteins can also combine with each other on the OCT4 gene to form a complex. For example, the transcription factor ZIC2 recruits nuclear remodeling factor (NURF) to form a complex in the OCT4 promoter region, activates OCT4 transcription, and promotes the self-renewal and differentiation potential of liver CSCs (85).
With the interaction between W118 residue of Ash2I and Oct4, Ash2I can further recruit other transcription factors Sox2 and Nanog to form the Ash2I/OSN complex in mESCs and activate downstream stem genes to jointly regulate the pluripotent network by epigenetic modifications (86). Increased ABCG2 (ATPase binding cassette transporter protein) in glioblastoma stem cells may interact with OCT4 to promote drug resistance and CSC survival (87, 88). The complexes formed by OCT4 may induce other pluripotency-related transcription factors to regulate the characteristics of CSCs. For example, the Oct4/Sox2/Klf4 complex binds to the Nanog promoter to induce its transcriptional activity and achieve the function of regulating stem cell-like characteristic in mESCs (89). Actually, some researchers have suggested that OCT4 is sufficient for pluripotential reprogramming of human neural stem cells (26).
OCT4 can independently perform stem-related regulation or form a complex with stem cell transcription factors or other functional proteins to regulate CSC proliferation, self-renewal, and invasion (Table 2). Therefore, how OCT4 interacts with binding proteins will become our research direction, which may provide new methods for our CSC treatment by interfering with complex formation or blocking regulatory networks.
In addition to being regulated by pluripotency-related transcription factors (such as OCT4, SOX2, NANOG, KLF4, MYC) and extracellular factors (such as hypoxia and extracellular matrix), CSCs are also interfered by various signaling pathways (such as Wnt, Notch, PI3K/Akt, JAK/STAT, Hedgehog) (90). Studies have found that OCT4 is also involved in the complex regulation of CSCs as an intermediate station or terminal target in the signaling pathway.
Hedgehog signaling pathway stimulated by the binding of Hh ligand and transmembrane protein receptor PTCH can regulate CSC metastasis and self-renewal ability by up-regulating the expression of downstream target gene OCT4 (52, 90). The lncRNA-Hh regulated by the gene TWIST, induces the expression of OCT4 by promoting the Hedeghog pathway and maintains the tumorigenicity and self-renewal ability of breast CSCs (52).
Increasing evidence suggests OCT4 is functionally dependent on signal transducer and activator of transcription 3 (STAT3) and the expression of OCT4 is positively related to the activity and expression of STAT3 in breast CSCs, cervical CSCs and liver CSCs (91-95). Inter-leukin 6 (IL-6) activates the Janus kinase1 (JAK1)/STAT3 signaling pathway and downstream OCT4 expression to complete the transformation of human breast cancer cells into breast CSCs (96, 97). The leukemia inhibitory factor (LIF) as a member of the IL-6 family can also activate this pathway (97). And IL-6 can also upregulate OCT4 through JAK2/STAT3 signaling to maintain the recu-rrence and drug resistance of liver CSCs (98). Interestingly, IL-6 also transiently upregulated protein tyrosine phosphatase receptor-type δ (PTPRD), which in turn dephosphorylates STAT3 and inhibits IL-6/STAT3 signal transduction and OCT4 expression in human breast cancer cells (99). At the same time, it was found that OCT4 can also activate the JAK1/STAT6 signaling pathway in ovarian CSCs and promote tumorigenesis (90). Moreover, OCT4 induces the production of IL-24 through the STAT3 and NF-κB signaling pathways, which can confer radiotherapy resistance to breast cancer cells by inhibiting radiation-induced senescence (65). The recognition and combination of ligand chemokine ligand 21 (CCL21) and C-C chemokine receptor 7 (CCR7) increase the expression of pluripotency-related transcription factors and stem cell markers (such as OCT4, CD133, CD44) by activating the JAK2/STAT3 signaling pathway, thereby enhancing the invasion, migration, and tumorsphere formation of oral squamous cell carcinoma (100). In addition, the STAT3 or STAT5A plays an important role in glioblastoma, which can be verified by the expression of OCT4 (101). The study further found that JAK/STAT3 activity is essential for DNA demethylation of Oct4 promoter during mouse somatic cell reprogramming (102, 103). Ganoderma lucidum extract (GLE) with anticancer activity inhibits the STAT3 signaling pathway thereby reducing the expression of phosphorylated and total STAT3 and OCT4 of breast CSCs (91). Ovatodiolide reduces the expression of genes, such as OCT4, and the formation of tumorspheres by inhibiting the JAK2/STAT3 signaling pathway, and cooperates with cisplatin to complete the treatment of oral cancer (104). Survivin is an apoptosis suppressor protein and is associated with chemoradiation resistance and metastasis of tumor cells (105). In hepatocellular adenocarcinoma, OCT4 was found to regulate the migration and invasion of cancer cells through the Survivin/STAT3 signaling pathway (106).
The PI3K/Akt pathway, which is related to cell growth and apoptosis, phosphorylates the substrate FOXO transcription factor to inactivate it and thereby reducing the expression of OCT4, because FOXO can directly bind to the OCT4 promoter to regulate OCT4 transcription, which indicates that targeting FOXO factors can reduce the generation of CSCs and the use of PI3K inhibitors clinically has potential risks (107-109). Likewise, phosphorylated Akt can phosphorylate OCT4, which increases tumorigenicity and self-renewal ability of CSCs (110). However, the researchers demonstrate that the Akt pathway is activated when mouse embryonic carcinoma cells begin to differentiate, and Akt phosphorylates Oct4 at serine 228 to accelerate its degradation (111).
The Wnt/β-catenin signaling pathway plays a huge role in maintaining the stem cell-like characteristics of breast CSCs and ESCs (112, 113). Diallyl Trisulfide can suppress Wnt/β-catenin signaling pathway and OCT4 expression to inhibit breast CSCs (112). Exogenous intake of bisphenol A (BPA) and polychlorinated biphenyls (PCBs) can increase the expression of stem cell markers, such as OCT4, and drug resistance of human ovarian cancer cells by activating Wnt/β-catenin pathway (114). OCT4 can also bind to enhancers of target genes activated by the Wnt/β-catenin signaling pathway, so the deletion of OCT4 has a direct influence on the Wnt/β-catenin signaling pathway (115). A contradictory result suggests that Wnt/β-catenin plays a role in hESC differentiation rather than self-renewal and that OCT4 may inhibit this pathway (116).
Studies have found that fine particulate matter (PM2.5) exposed to human lung cancer cells promotes the expression of OCT4 by activating the Notch signaling pathway, thereby promoting the occurrence of lung CSCs (117). Similarly, the relationship between the Notch pathway and OCT4 has also been confirmed in pancreatic CSCs (118). Evidence suggests that the c-Met signaling pathway promotes the self-renewal and metastasis of glioblastoma stem cells by up-regulating the expression of OCT4 and c-MYC (119). TGF-β RI is highly expressed in tissues with highly metastatic endometriosis, and it was found that the completion of TGF-β signal transduction requires the addition of OCT4 (120). Moreover, the knockout of OCT4 in liver cancer cells significantly reduces the expression of genes related to the TGF-β pathway (ELF, Smad3, Smad4), which indicates that OCT4 plays an important role in CSCs by improving the TGF-β pathway (121). The Hippo signaling pathway was found to be inhibited during the progression from colorectal adenoma to colorectal cancer, and OCT4 as a target gene of the Hippo pathway was upregulated in the case of overexpression of downstream cascade kinases, which may be related to the progression and metastasis of colorectal cancer (122). The pluripotency mediator b-FGF upregulates the expression of OCT4 and maintains the undifferentiated state of human induced pluripotent stem cells through the mitogen-activated protein kinase (MAPK) signaling pathway (NRAS-RAF-MEK-ERK) (123).
The various signaling pathways are not independent, and they may be synergistic in regulating OCT4 expression. Argonaute 2/OCT4/methyl-CpG-binding protein 6 (Ago2/OCT4/MBD6) signal transduction pathway was found to regulate stemness-related genes and human adipose tissue-derived stem cell self-renewal (124). In addition, Ago2 can also regulate human umbilical cord blood-derived me-senchymal stem cell self-renewal through the expression of OCT4 and activation of Wnt/β-catenin and JAK2/STAT3 signaling pathways (125). IL-17B related to cancer progression enhances the expression of OCT4, SOX2 and other transcription factors and activates the NF-κΒ, STAT3 and β-catenin pathways to promote the progre-ssion of gastric cancer (126). IL-23, which is positively correlated with OCT4 expression, promotes the self-renewal ability and tumorigenicity potential of ovarian CSCs through NF-κΒ and STAT3 signaling pathways (127). The PI3K/Akt2/mTOR signaling pathway and MAPK signaling pathway promote the cisplatin and radiation resistance of neuroblastoma cells by regulating the expression of OCT4, SOX2, CD133 and ALDH (128). In addition to the PTEN/PI3K/AKT/β-catenin axis, miR-429, which is hypomethylation and highly expressed in liver CSCs, also regulates the generation, invasion and metastasis of liver cancer through the Rb binding protein 4/E2F transcription factor 1/OCT4 (RBBP4/E2F1/OCT4) axis (129, 130). Phosphorylation and inactivation of GSK3β by Wnt, PI3K, Akt, and MAPK pathway reduce the expression of OCT4, but in head and neck cancer, CD44 can inhibit Akt phosphorylation and thus inhibit GSK3β inactivation and maintain the self-renewal ability of CSCs (131).
In fact, OCT4-related signaling pathways are complex in maintaining the stem characteristics of CSCs (Fig. 2). There may be conflicting research results on the same signal pathway, which requires us to further determine whether there is a difference in cancer species specificity or cellular time limit. The discovery of signal pathways related to OCT4 provides many targets for radical treatment of CSCs, which may well solve the problems of cancer recurrence, metastasis and drug resistance.
The role of OCT4 in pluripotency maintenance makes OCT4 a new cancer treatment target. Increased experiments have found that siRNA targeting OCT4 induces apoptosis, inhibits proliferation, EMT, and drug resistance in pancreatic cancer cells and head and neck squamous cell carcinoma CSCs, breast CSCs (66-68). Similar results have been found in lung cancer, ovarian cancer, liver cancer, and glioma (37, 106). However, an experiment suggested that OCT4 knockout in the MCF-7 human breast cancer cell line induced cell invasion, migration, and EMT, which is because MCF-7 cells already have high expression of OCT4 (37, 132). Moreover, due to the intra- and extracellular degradation of enzymes, the delivery efficiency of siRNA is limited, which still needs to be solved (66). The epigenetic regulator JMJD3 inhibits OCT4 expression in human breast cancer cells in an independent manner with demethylase activity, and paricalcitol, a vitamin D analog, inhibits OCT4 expression and stem cell-like characteristics of human breast cancer cells after promoting JMJD3 expression (133). Recently, three different Oct4 epitopes were chemically synthesized, among which Oct4-3 and carrier protein KLH induced a strong tumor-specific adaptive immune response in combination with toll-like receptor 9 agonist, thereby inhibiting mouse testis embryonic carcinoma growth and promoting long-term survival. Importantly, the mice were well tolerated with the Oct4-3 vaccine and no obvious adverse events were observed (134). Additionally, targeting upstream activators of OCT4 is also a new method. For instance, the use of Notch pathway inhibitor L685,458 can reduce the expression of OCT4 and reverse stem cell-like phenotype and resistance to paclitaxel of breast cancer cells (19, 135). Using siRNA to target OCT4B1 induces apoptosis or G2/M arrest in human brain cancer cells, suggesting that OCT4B1 may also be a potential therapeutic target in brain cancer (136).
CSCs are a group of cells with the ability to self-renew and differentiate among cancer cells. Their discovery provides the possibility to solve tumor recurrence, metastasis and resistance. OCT4 as a stem transcription factor is widely expressed in ESCs and CSCs, and research has found that OCT4 may participate in the regulation of CSCs through various forms, but the specific mechanism may not be clear. This review mainly proposes the specific role of OCT4 in CSCs from the epigenetic modification of OCT4 and complexes, non-coding RNA and signaling pathways related to OCT4, which may provide multiple targets for the treatment of CSCs to achieve the purpose of extending patient life.
Acknowledgments
This review was supported by the National Natural Science Foundation of China (81372835 and 81670143), and National Key Research and Development Program of China (2018YFA0106902). We thank all the authors for their valuable suggestions.
References
1. Pozzi V, Salvolini E, Lucarini G, Salvucci A, Campagna R, Rubini C, Sartini D, Emanuelli M. 2020; Cancer stem cell enrichment is associated with enhancement of nicotinamide N-methyltransferase expression. IUBMB Life. 72:1415–1425. DOI: 10.1002/iub.2265. PMID: 32150326.

2. Unver N. 2020; Cancer stemness as a target for immunotherapy is shaped by pro-inflammatory stress. Curr Stem Cell Res Ther. doi:10.2174/1574888X15666200309145901. [Epub ahead of print]. DOI: 10.2174/1574888X15666200309145901. PMID: 32148202.

3. Mortezaee K. 2020; CXCL12/CXCR4 axis in the microenvironment of solid tumors: a critical mediator of metastasis. Life Sci. 249:117534. DOI: 10.1016/j.lfs.2020.117534. PMID: 32156548.

4. Tahmasebi E, Alikhani M, Yazdanian A, Yazdanian M, Tebyanian H, Seifalian A. 2020; The current markers of cancer stem cell in oral cancers. Life Sci. 249:117483. DOI: 10.1016/j.lfs.2020.117483. PMID: 32135187.

5. Bigdelou Z, Mortazavi Y, Saltanatpour Z, Asadi Z, Kadivar M, Johari B. 2020; Role of Oct4-Sox2 complex decoy oligodeoxynucleotides strategy on reverse epithelial to mesenchymal transition (EMT) induction in HT29-ShE encompassing enriched cancer stem-like cells. Mol Biol Rep. 47:1859–1869. DOI: 10.1007/s11033-020-05280-2. PMID: 32016633.

6. Zhao Y, Li C, Huang L, Niu S, Lu Q, Gong D, Huang S, Yuan Y, Chen H. 2018; Prognostic value of association of OCT4 with LEF1 expression in esophageal squamous cell carcinoma and their impact on epithelial-mesenchymal transition, invasion, and migration. Cancer Med. 7:3977–3987. DOI: 10.1002/cam4.1641. PMID: 29974668. PMCID: PMC6089166.

7. Zeineddine D, Hammoud AA, Mortada M, Boeuf H. 2014; The Oct4 protein: more than a magic stemness marker. Am J Stem Cells. 3:74–82. PMID: 25232507. PMCID: PMC4163606.
8. van Schaijik B, Davis PF, Wickremesekera AC, Tan ST, Itinteang T. 2018; Subcellular localisation of the stem cell markers OCT4, SOX2, NANOG, KLF4 and c-MYC in cancer: a review. J Clin Pathol. 71:88–91. DOI: 10.1136/jclinpath-2017-204815. PMID: 29180509.

9. Shi G, Jin Y. 2010; Role of Oct4 in maintaining and regaining stem cell pluripotency. Stem Cell Res Ther. 1:39. DOI: 10.1186/scrt39. PMID: 21156086. PMCID: PMC3025441.

10. Okamoto K, Okazawa H, Okuda A, Sakai M, Muramatsu M, Hamada H. 1990; A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 60:461–472. DOI: 10.1016/0092-8674(90)90597-8. PMID: 1967980.

11. Schöler HR, Ruppert S, Suzuki N, Chowdhury K, Gruss P. 1990; New type of POU domain in germ line-specific protein Oct-4. Nature. 344:435–439. DOI: 10.1038/344435a0. PMID: 1690859.

12. Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby PW, Staudt LM. 1990; A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 345:686–692. DOI: 10.1038/345686a0. PMID: 1972777.

13. Kim JB, Sebastiano V, Wu G, Araúzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, Meyer J, Hübner K, Bernemann C, Ortmeier C, Zenke M, Fleischmann BK, Zaehres H, Schöler HR. 2009; Oct4-induced pluripotency in adult neural stem cells. Cell. 136:411–419. DOI: 10.1016/j.cell.2009.01.023. PMID: 19203577.

14. Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. 2005; Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 26:495–502. DOI: 10.1093/carcin/bgh321. PMID: 15513931.

15. Shao M, Bi T, Ding W, Yu C, Jiang C, Yang H, Sun X, Yang M. 2018; OCT4 potentiates radio-resistance and migration activity of rectal cancer cells by improving epithelial-mesenchymal transition in a ZEB1 dependent manner. Biomed Res Int. 2018:3424956. DOI: 10.1155/2018/3424956. PMID: 30112378. PMCID: PMC6077687.

16. Zhao FQ. 2013; Octamer-binding transcription factors: genomics and functions. Front Biosci (Landmark Ed). 18:1051–1071. DOI: 10.2741/4162. PMID: 23747866. PMCID: PMC4349413.

17. Huertas J, MacCarthy CM, Schöler HR, Cojocaru V. 2020; Nucleosomal DNA dynamics mediate Oct4 pioneer factor binding. Biophys J. 118:2280–2296. DOI: 10.1016/j.bpj.2019.12.038. PMID: 32027821. PMCID: PMC7202942.

18. Medvedev SP, Shevchenko AI, Elisaphenko EA, Nesterova TB, Brockdorff N, Zakian SM. 2008; Structure and expression pattern of Oct4 gene are conserved in vole Microtus rossiaemeridionalis. BMC Genomics. 9:162. DOI: 10.1186/1471-2164-9-162. PMID: 18402712. PMCID: PMC2410140.

19. Mohiuddin IS, Wei SJ, Kang MH. 2020; Role of OCT4 in cancer stem-like cells and chemotherapy resistance. Biochim Biophys Acta Mol Basis Dis. 1866:165432. DOI: 10.1016/j.bbadis.2019.03.005. PMID: 30904611. PMCID: PMC6754810.

20. Wu G, Wilson G, Zhou G, Hebbard L, George J, Qiao L. 2015; Oct4 is a reliable marker of liver tumor propagating cells in hepatocellular carcinoma. Discov Med. 20:219–229. PMID: 26562475.
21. Hatefi N, Nouraee N, Parvin M, Ziaee SA, Mowla SJ. 2012; Evaluating the expression of oct4 as a prognostic tumor marker in bladder cancer. Iran J Basic Med Sci. 15:1154–1161. PMID: 23653844. PMCID: PMC3646225.
22. Chen Y, Li XG. 2006; Epigenetic modification in human leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 14:635–638. Chinese.
23. Juárez-Moreno K, Erices R, Beltran AS, Stolzenburg S, Cuello-Fredes M, Owen GI, Qian H, Blancafort P. 2013; Breaking through an epigenetic wall: re-activation of Oct4 by KRAB-containing designer zinc finger transcription factors. Epi-genetics. 8:164–176. DOI: 10.4161/epi.23503. PMID: 23314702. PMCID: PMC3592902.
24. Kristensen DM, Nielsen JE, Kalisz M, Dalgaard MD, Audouze K, Larsen ME, Jacobsen GK, Horn T, Brunak S, Skakkebaek NE, Leffers H. 2010; OCT4 and downstream factors are expressed in human somatic urogenital epithelia and in culture of epididymal spheres. Mol Hum Reprod. 16:835–845. DOI: 10.1093/molehr/gaq008. PMID: 20123703.

25. Zhang HJ, Siu MK, Wong ES, Wong KY, Li AS, Chan KY, Ngan HY, Cheung AN. 2008; Oct4 is epigenetically regulated by methylation in normal placenta and gestational trophoblastic disease. Placenta. 29:549–554. DOI: 10.1016/j.placenta.2008.03.003. PMID: 18440631.

26. Zhao HX, Li Y, Jin HF, Xie L, Liu C, Jiang F, Luo YN, Yin GW, Li Y, Wang J, Li LS, Yao YQ, Wang XH. 2010; Rapid and efficient reprogramming of human amnion-derived cells into pluripotency by three factors OCT4/SOX2/NAN OG. Differentiation. 80:123–129. DOI: 10.1016/j.diff.2010.03.002. PMID: 20510497.

27. Hattori N, Nishino K, Ko YG, Hattori N, Ohgane J, Tanaka S, Shiota K. 2004; Epigenetic control of mouse Oct-4 gene expression in embryonic stem cells and trophoblast stem cells. J Biol Chem. 279:17063–17069. DOI: 10.1074/jbc.M309002200. PMID: 14761969.

28. Liu Q, Chen K, Liu Z, Huang Y, Zhao R, Wei L, Yu X, He J, Liu J, Qi J, Qin Y, Li B. 2017; BORIS up-regulates OCT4 via histone methylation to promote cancer stem cell-like properties in human liver cancer cells. Cancer Lett. 403:165–174. DOI: 10.1016/j.canlet.2017.06.017. PMID: 28645561.

29. Dai X, Liu P, Lau AW, Liu Y, Inuzuka H. 2014; Acetylation-dependent regulation of essential iPS-inducing factors: a regulatory crossroad for pluripotency and tumorigenesis. Cancer Med. 3:1211–1224. DOI: 10.1002/cam4.298. PMID: 25116380. PMCID: PMC4302671.

30. Chai S, Xu X, Wang Y, Zhou Y, Zhang C, Yang Y, Yang Y, Xu H, Xu R, Wang K. 2015; Ca2+/calmodulin-dependent protein kinase IIγ enhances stem-like traits and tumorigenicity of lung cancer cells. Oncotarget. 6:16069–16083. DOI: 10.18632/oncotarget.3866. PMID: 25965829. PMCID: PMC4599257.

31. Tan Y, Xue Y, Song C, Grunstein M. 2013; Acetylated histone H3K56 interacts with Oct4 to promote mouse embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 110:11493–11498. DOI: 10.1073/pnas.1309914110. PMID: 23798425. PMCID: PMC3710873.

32. Guo L, Zhou Y, Wang S, Wu Y. 2014; Epigenetic changes of mesenchymal stem cells in three-dimensional (3D) sphe-roids. J Cell Mol Med. 18:2009–2019. DOI: 10.1111/jcmm.12336. PMID: 25090911. PMCID: PMC4244016.

33. Abulaiti X, Zhang H, Wang A, Li N, Li Y, Wang C, Du X, Li L. 2017; Phosphorylation of threonine343 is crucial for OCT4 interaction with SOX2 in the maintenance of mouse embryonic stem cell pluripotency. Stem Cell Reports. 9:1630–1641. DOI: 10.1016/j.stemcr.2017.09.001. PMID: 28988986. PMCID: PMC5829306.

34. Deng L, Meng T, Chen L, Wei W, Wang P. 2020; The role of ubiquitination in tumorigenesis and targeted drug disco-very. Signal Transduct Target Ther. 5:11. DOI: 10.1038/s41392-020-0107-0. PMID: 32296023. PMCID: PMC7048745.

35. Liao B, Zhong X, Xu H, Xiao F, Fang Z, Gu J, Chen Y, Zhao Y, Jin Y. 2013; Itch, an E3 ligase of Oct4, is required for embryonic stem cell self-renewal and pluripotency induction. J Cell Physiol. 228:1443–1451. DOI: 10.1002/jcp.24297. PMID: 23255053.

36. Cho Y, Kang HG, Kim SJ, Lee S, Jee S, Ahn SG, Kang MJ, Song JS, Chung JY, Yi EC, Chun KH. 2018; Post-translational modification of OCT4 in breast cancer tumorigene-sis. Cell Death Differ. 25:1781–1795. DOI: 10.1038/s41418-018-0079-6. PMID: 29511337. PMCID: PMC6180041.

37. Villodre ES, Kipper FC, Pereira MB, Lenz G. 2016; Roles of OCT4 in tumorigenesis, cancer therapy resistance and prognosis. Cancer Treat Rev. 51:1–9. DOI: 10.1016/j.ctrv.2016.10.003. PMID: 27788386.

38. Jang H, Kim TW, Yoon S, Choi SY, Kang TW, Kim SY, Kwon YW, Cho EJ, Youn HD. 2012; O-GlcNAc regulates pluripotency and reprogramming by directly acting on core components of the pluripotency network. Cell Stem Cell. 11:62–74. DOI: 10.1016/j.stem.2012.03.001. PMID: 22608532.

39. Lou W, Ding B, Fu P. 2020; Pseudogene-derived lncRNAs and their miRNA sponging mechanism in human cancer. Front Cell Dev Biol. 8:85. DOI: 10.3389/fcell.2020.00085. PMID: 32185172. PMCID: PMC7058547.

40. Chen Q, Zhu C, Jin Y, Si X, Jiao W, He W, Mao W, Li M, Luo G. 2020; Plasma long non-coding RNA RP11-438N5.3 as a novel biomarker for non-small cell lung cancer. Cancer Manag Res. 12:1513–1521. DOI: 10.2147/CMAR.S237024. PMID: 32184656. PMCID: PMC7055527.
41. Zhu Y, Luo C, Korakkandan AA, Fatma YHA, Tao Y, Yi T, Hu S, Liao Q. 2020; Function and regulation annotation of up-regulated long non-coding RNA LINC01234 in gastric cancer. J Clin Lab Anal. 34:e23210. DOI: 10.1002/jcla.23210. PMID: 32011780. PMCID: PMC7246363.

42. Guo QS, Wang P, Huang Y, Guo YB, Zhu MY, Xiong YC. 2019; Regulatory effect of miR-30b on migration and invasion of pancreatic cancer stem cells. Zhonghua Yi Xue Za Zhi. 99:3019–3023. Chinese.
43. Guo JC, Yang YJ, Zheng JF, Zhang JQ, Guo M, Yang X, Jiang XL, Xiang L, Li Y, Ping H, Zhuo L. 2019; Silencing of long noncoding RNA HOXA11-AS inhibits the Wnt signaling pathway via the upregulation of HOXA11 and thereby inhibits the proliferation, invasion, and self-renewal of hepatocellular carcinoma stem cells. Exp Mol Med. 51:1–20. DOI: 10.1038/s12276-019-0328-x. PMCID: PMC6874533. PMID: 31757938.

44. Zhao Y, Zhu Z, Shi S, Wang J, Li N. 2019; Long non-coding RNA MEG3 regulates migration and invasion of lung cancer stem cells via miR-650/SLC34A2 axis. Biomed Phar-macother. 120:109457. DOI: 10.1016/j.biopha.2019.109457. PMID: 31585300.

45. Zhao W, Li W, Jin X, Niu T, Cao Y, Zhou P, Zheng M. 2019; Silencing long non-coding RNA NEAT1 enhances the suppression of cell growth, invasion, and apoptosis of bladder cancer cells under cisplatin chemotherapy. Int J Clin Exp Pathol. 12:549–558. PMID: 31933859. PMCID: PMC6945077.
46. Xu Z, Liu C, Zhao Q, Lü J, Ding X, Luo A, He J, Wang G, Li Y, Cai Z, Wang Z, Liu J, Liu S, Li W, Yu Z. 2020; Long non-coding RNA CCAT2 promotes oncogenesis in triple-negative breast cancer by regulating stemness of cancer cells. Pharmacol Res. 152:104628. DOI: 10.1016/j.phrs.2020.104628. PMID: 31904506.

47. Tang D, Yang Z, Long F, Luo L, Yang B, Zhu R, Sang X, Cao G, Wang K. 2019; Long noncoding RNA MALAT1 mediates stem cell-like properties in human colorectal cancer cells by regulating miR-20b-5p/Oct4 axis. J Cell Physiol. 234:20816–20828. DOI: 10.1002/jcp.28687. PMID: 31012108.

48. Fan H, Liu G, Zhao C, Li X, Yang X. 2017; Transcription factor Oct4 promotes osteosarcoma by regulating lncRNA AK055 347. Oncol Lett. 13:396–402. DOI: 10.3892/ol.2016.5400. PMID: 28123573. PMCID: PMC5244871.

49. Han Q, Xu L, Lin W, Yao X, Jiang M, Zhou R, Sun X, Zhao L. 2019; Long noncoding RNA CRCMSL suppresses tumor invasive and metastasis in colorectal carcinoma through nucleocytoplasmic shuttling of HMGB2. Oncogene. 38:3019–3032. DOI: 10.1038/s41388-018-0614-4. PMID: 30575817.

50. Bauderlique-Le Roy H, Vennin C, Brocqueville G, Spruyt N, Adriaenssens E, Bourette RP. 2015; Enrichment of human stem-like prostate cells with s-SHIP promoter activity uncovers a role in stemness for the long noncoding RNA H19. Stem Cells Dev. 24:1252–1262. DOI: 10.1089/scd.2014.0386. PMID: 25567531. PMCID: PMC4425227.

51. Zimmerman DL, Boddy CS, Schoenherr CS. 2013; Oct4/Sox2 binding sites contribute to maintaining hypomethylation of the maternal igf2/h19 imprinting control region. PLoS One. 8:e81962. DOI: 10.1371/journal.pone.0081962. PMID: 24324735. PMCID: PMC3855764.

52. Chen S, Zhu J, Wang F, Guan Z, Ge Y, Yang X, Cai J. 2017; LncRNAs and their role in cancer stem cells. Oncotarget. 8:110685–110692. DOI: 10.18632/oncotarget.22161. PMID: 29299179. PMCID: PMC5746414.

53. Wang Y, Xu Z, Jiang J, Xu C, Kang J, Xiao L, Wu M, Xiong J, Guo X, Liu H. 2013; Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev Cell. 25:69–80. DOI: 10.1016/j.devcel.2013.03.002. PMID: 23541921.

54. Sandmaier SE, Telugu BP. 2015; MicroRNA-mediated reprogra-mming of somatic cells into induced pluripotent stem cells. Methods Mol Biol. 1330:29–36. DOI: 10.1007/978-1-4939-2848-4_3. PMID: 26621586.

55. Bräutigam C, Raggioli A, Winter J. 2013; The Wnt/β-catenin pathway regulates the expression of the miR-302 cluster in mouse ESCs and P19 cells. PLoS One. 8:e75315. DOI: 10.1371/journal.pone.0075315. PMID: 24040406. PMCID: PMC3769259.
56. Hu S, Wilson KD, Ghosh Z, Han L, Wang Y, Lan F, Ransohoff KJ, Burridge P, Wu JC. 2013; MicroRNA-302 increases reprogramming efficiency via repression of NR2F2. Stem Cells. 31:259–268. DOI: 10.1002/stem.1278. PMID: 23136034. PMCID: PMC3572288.

57. Wu Y, Liu S, Xin H, Jiang J, Younglai E, Sun S, Wang H. 2011; Up-regulation of microRNA-145 promotes differentia-tion by repressing OCT4 in human endometrial adenocar-cinoma cells. Cancer. 117:3989–3998. DOI: 10.1002/cncr.25944. PMID: 21365617.

58. Yang YP, Chien Y, Chiou GY, Cherng JY, Wang ML, Lo WL, Chang YL, Huang PI, Chen YW, Shih YH, Chen MT, Chiou SH. 2012; Inhibition of cancer stem cell-like properties and reduced chemoradioresistance of glioblastoma using microRNA145 with cationic polyurethane-short branch PEI. Biomaterials. 33:1462–1476. DOI: 10.1016/j.biomaterials.2011.10.071. PMID: 22098779.

59. Jerabek S, Merino F, Schöler HR, Cojocaru V. 2014; OCT4: dynamic DNA binding pioneers stem cell pluripotency. Biochim Biophys Acta. 1839:138–154. DOI: 10.1016/j.bbagrm.2013.10.001. PMID: 24145198.

60. Gao Y, Zhang Z, Li K, Gong L, Yang Q, Huang X, Hong C, Ding M, Yang H. 2017; Linc-DYNC2H1-4 promotes EMT and CSC phenotypes by acting as a sponge of miR-145 in pancreatic cancer cells. Cell Death Dis. 8:e2924. DOI: 10.1038/cddis.2017.311. PMID: 28703793. PMCID: PMC5550858.

61. Bai M, Yuan M, Liao H, Chen J, Xie B, Yan D, Xi X, Xu X, Zhang Z, Feng Y. 2015; OCT4 pseudogene 5 upregulates OCT4 expression to promote proliferation by competing with miR-145 in endometrial carcinoma. Oncol Rep. 33:1745–1752. DOI: 10.3892/or.2015.3763. PMID: 25634023.

62. Wang L, Guo ZY, Zhang R, Xin B, Chen R, Zhao J, Wang T, Wen WH, Jia LT, Yao LB, Yang AG. 2013; Pseudogene OCT4-pg4 functions as a natural micro RNA sponge to regulate OCT4 expression by competing for miR-145 in hepatocellular carcinoma. Carcinogenesis. 34:1773–1781. DOI: 10.1093/carcin/bgt139. PMID: 23615404.

63. Liu T, Chi H, Chen J, Chen C, Huang Y, Xi H, Xue J, Si Y. 2017; Curcumin suppresses proliferation and in vitro invasion of human prostate cancer stem cells by ceRNA effect of miR-145 and lncRNA-ROR. Gene. 631:29–38. DOI: 10.1016/j.gene.2017.08.008. PMID: 28843521.

64. Chai S, Ng KY, Tong M, Lau EY, Lee TK, Chan KW, Yuan YF, Cheung TT, Cheung ST, Wang XQ, Wong N, Lo CM, Man K, Guan XY, Ma S. 2016; Octamer 4/microRNA-1246 signaling axis drives Wnt/β-catenin activation in liver cancer stem cells. Hepatology. 64:2062–2076. DOI: 10.1002/hep.28821. PMID: 27639189.

65. Kim JY, Kim JC, Lee JY, Park MJ. 2018; Oct4 suppresses IR-induced premature senescence in breast cancer cells through STAT3- and NF-κB-mediated IL‑24 production. Int J Oncol. 53:47–58. DOI: 10.3892/ijo.2018.4391. PMID: 29749438. PMCID: PMC5958730.
66. Lo WL, Chien Y, Chiou GY, Tseng LM, Hsu HS, Chang YL, Lu KH, Chien CS, Wang ML, Chen YW, Huang PI, Hu FW, Yu CC, Chu PY, Chiou SH. 2012; Nuclear localization signal-enhanced RNA interference of EZH2 and Oct4 in the eradication of head and neck squamous cell carcinoma-derived cancer stem cells. Biomaterials. 33:3693–3709. DOI: 10.1016/j.biomaterials.2012.01.016. PMID: 22361100.

67. Huang JQ. 2011; Small interfering RNA-mediated OCT4 gene silencing inhibits the proliferation and induces apoptosis of pancreatic cancer cell line PANC1. Nan Fang Yi Ke Da Xue Xue Bao. 31:860–863. Chinese.
68. Huang ZJ, You J, Luo WY, Chen BS, Feng QZ, Wu BL, Jiang L, Luo Q. 2015; Reduced tumorigenicity and drug resistance through the downregulation of octamer-binding protein 4 and Nanog transcriptional factor expression in human breast stem cells. Mol Med Rep. 11:1647–1654. DOI: 10.3892/mmr.2014.2972. PMID: 25405855. PMCID: PMC4270319.

69. Mydlikova Z, Gursky J, Pirsel M. 2010; Transcription factor IIH-the protein complex with multiple functions. Neoplasma. 57:287–290. DOI: 10.4149/neo_2010_04_287. PMID: 20429618.
70. Whitton B, Okamoto H, Packham G, Crabb SJ. 2018; Vacuolar ATPase as a potential therapeutic target and mediator of treatment resistance in cancer. Cancer Med. 7:3800–3811. DOI: 10.1002/cam4.1594. PMID: 29926527. PMCID: PMC6089187.

71. Bourguignon LYW, Earle C, Shiina M. 2017; Activation of matrix hyaluronan-mediated CD44 signaling, epigenetic regulation and chemoresistance in head and neck cancer stem cells. Int J Mol Sci. 18:1849. DOI: 10.3390/ijms18091849. PMID: 28837080. PMCID: PMC5618498.

72. Fu TY, Hsieh IC, Cheng JT, Tsai MH, Hou YY, Lee JH, Liou HH, Huang SF, Chen HC, Yen LM, Tseng HH, Ger LP. 2016; Association of OCT4, SOX2, and NANOG expression with oral squamous cell carcinoma progression. J Oral Pathol Med. 45:89–95. DOI: 10.1111/jop.12335. PMID: 26211876.

73. Pan X, Cang X, Dan S, Li J, Cheng J, Kang B, Duan X, Shen B, Wang YJ. 2016; Site-specific disruption of the Oct4/Sox2 protein interaction reveals coordinated mesendodermal differentiation and the epithelial-mesenchymal transition. J Biol Chem. 291:18353–18369. DOI: 10.1074/jbc.M116.745414. PMID: 27369080. PMCID: PMC5000082.

74. Herreros-Villanueva M, Bujanda L, Billadeau DD, Zhang JS. 2014; Embryonic stem cell factors and pancreatic cancer. World J Gastroenterol. 20:2247–2254. DOI: 10.3748/wjg.v20.i9.2247. PMID: 24605024. PMCID: PMC3942830.

75. Liang Y, Huimei Hong F, Ganesan P, Jiang S, Jauch R, Stanton LW, Kolatkar PR. 2012; Structural analysis and dimerization profile of the SCAN domain of the pluripotency factor Zfp206. Nucleic Acids Res. 40:8721–8732. DOI: 10.1093/nar/gks611. PMID: 22735705. PMCID: PMC3458555.

76. Zhao B, Zheng X, Tan X, Ke K, Wang F, Wang Y, Xing X, Zhang C, Hu P, Lan S, Li Q, Huang A, Liu X. 2020; Ku80 negatively regulates the expression of OCT4 via competitive binding to SALL4 and promoting lysosomal degradation of OCT4. Int J Biochem Cell Biol. 118:105664. DOI: 10.1016/j.biocel.2019.105664. PMID: 31816404.

77. Zhang X, Yuan X, Zhu W, Qian H, Xu W. 2015; SALL4: an emerging cancer biomarker and target. Cancer Lett. 357:55–62. DOI: 10.1016/j.canlet.2014.11.037. PMID: 25444934.

78. Tanimura N, Saito M, Ebisuya M, Nishida E, Ishikawa F. 2013; Stemness-related factor Sall4 interacts with transcription factors Oct-3/4 and Sox2 and occupies Oct-Sox elements in mouse embryonic stem cells. J Biol Chem. 288:5027–5038. DOI: 10.1074/jbc.M112.411173. PMID: 23269686. PMCID: PMC3576104.

79. Maiuthed A, Bhummaphan N, Luanpitpong S, Mutirangura A, Aporntewan C, Meeprasert A, Rungrotmongkol T, Rojanasakul Y, Chanvorachote P. 2018; Nitric oxide promotes cancer cell dedifferentiation by disrupting an Oct4:caveolin-1 complex: a new regulatory mechanism for cancer stem cell formation. J Biol Chem. 293:13534–13552. DOI: 10.1074/jbc.RA117.000287. PMID: 29986880. PMCID: PMC6120192.

80. Yoon HJ, Kim DH, Kim SJ, Jang JH, Surh YJ. 2019; Src-mediated phosphorylation, ubiquitination and degradation of Caveolin-1 promotes breast cancer cell stemness. Cancer Lett. 449:8–19. DOI: 10.1016/j.canlet.2019.01.021. PMID: 30673589.

81. Yongsanguanchai N, Pongrakhananon V, Mutirangura A, Rojanasakul Y, Chanvorachote P. 2015; Nitric oxide induces cancer stem cell-like phenotypes in human lung cancer cells. Am J Physiol Cell Physiol. 308:C89–C100. DOI: 10.1152/ajpcell.00187.2014. PMID: 25411331.

82. Yang YC, Chien MH, Liu HY, Chang YC, Chen CK, Lee WJ, Kuo TC, Hsiao M, Hua KT, Cheng TY. 2018; Nuclear translocation of PKM2/AMPK complex sustains cancer stem cell populations under glucose restriction stress. Cancer Lett. 421:28–40. DOI: 10.1016/j.canlet.2018.01.075. PMID: 29408265.

83. Giannoni E, Taddei ML, Morandi A, Comito G, Calvani M, Bianchini F, Richichi B, Raugei G, Wong N, Tang D, Chiarugi P. 2015; Targeting stromal-induced pyruvate kinase M2 nuclear translocation impairs oxphos and prostate cancer metastatic spread. Oncotarget. 6:24061–24074. DOI: 10.18632/oncotarget.4448. PMID: 26183399. PMCID: PMC4695170.

84. Lei I, Tian S, Chen V, Zhao Y, Wang Z. 2020; SWI/SNF component BAF250a coordinates OCT4 and WNT signaling pathway to control cardiac lineage differentiation. Front Cell Dev Biol. 7:358. DOI: 10.3389/fcell.2019.00358. PMID: 32039194. PMCID: PMC6987383.

85. Zhu P, Wang Y, He L, Huang G, Du Y, Zhang G, Yan X, Xia P, Ye B, Wang S, Hao L, Wu J, Fan Z. 2015; ZIC2-dependent OCT4 activation drives self-renewal of human liver cancer stem cells. J Clin Invest. 125:3795–3808. DOI: 10.1172/JCI81979. PMID: 26426078. PMCID: PMC4607118.

86. Tsai PH, Chien Y, Wang ML, Hsu CH, Laurent B, Chou SJ, Chang WC, Chien CS, Li HY, Lee HC, Huo TI, Hung JH, Chen CH, Chiou SH. 2019; Ash2l interacts with Oct4-stemness circuitry to promote super-enhancer-driven pluripotency network. Nucleic Acids Res. 47:10115–10133. DOI: 10.1093/nar/gkz801. PMID: 31555818. PMCID: PMC6821267.

87. Chen D. 2015; Tumor formation and drug resistance properties of human glioblastoma side population cells. Mol Med Rep. 11:4309–4314. DOI: 10.3892/mmr.2015.3279. PMID: 25633829.

88. Blum W, Pecze L, Felley-Bosco E, Wu L, de Perrot M, Schwaller B. 2017; Stem cell factor-based identification and functional properties of in vitro-selected subpopulations of malignant mesothelioma cells. Stem Cell Reports. 8:1005–1017. DOI: 10.1016/j.stemcr.2017.02.005. PMID: 28285878. PMCID: PMC5390099.

89. Lee S, Wottrich S, Bonavida B. 2017; Crosstalks between Raf-kinase inhibitor protein and cancer stem cell transcription factors (Oct4, KLF4, Sox2, Nanog). Tumour Biol. 39:1010428317692253. DOI: 10.1177/1010428317692253. PMID: 28378634.

90. Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, Wang X, Dong Z, Chen F, Cui H. 2020; Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 5:8. DOI: 10.1038/s41392-020-0110-5. PMID: 32296030. PMCID: PMC7005297.

91. Rios-Fuller TJ, Ortiz-Soto G, Lacourt-Ventura M, Maldonado-Martinez G, Cubano LA, Schneider RJ, Martinez-Montemayor MM. 2018; Ganoderma lucidum extract (GLE) impairs breast cancer stem cells by targeting the STAT3 pathway. Oncotarget. 9:35907–35921. DOI: 10.18632/oncotarget.26294. PMID: 30542507. PMCID: PMC6267592.

92. Wang H, Deng J, Ren HY, Jia P, Zhang W, Li MQ, Li SW, Zhou QH. 2017; STAT3 influences the characteristics of stem cells in cervical carcinoma. Oncol Lett. 14:2131–2136. DOI: 10.3892/ol.2017.6454. PMID: 28781654. PMCID: PMC5530137.

93. Wang H, Cai HB, Chen LL, Zhao WJ, Li P, Wang ZQ, Li Z. 2015; STAT3 correlates with stem cell-related transcription factors in cervical cancer. J Huazhong Univ Sci Technolog Med Sci. 35:891–897. DOI: 10.1007/s11596-015-1524-0. PMID: 26670442.

94. Yang CM, Chiba T, Groner B. 2012; Expression of reprogramming factors in breast cancer cell lines and the regulation by activated Stat3. Horm Mol Biol Clin Investig. 10:241–248. DOI: 10.1515/hmbci-2012-0003. PMID: 25436680.

95. Yin X, Zhang BH, Zheng SS, Gao DM, Qiu SJ, Wu WZ, Ren ZG. 2015; Coexpression of gene Oct4 and Nanog initiates stem cell characteristics in hepatocellular carcinoma and promotes epithelial-mesenchymal transition through activation of Stat3/Snail signaling. J Hematol Oncol. 8:23. DOI: 10.1186/s13045-015-0119-3. PMID: 25879771. PMCID: PMC4377043.

96. Kim SY, Kang JW, Song X, Kim BK, Yoo YD, Kwon YT, Lee YJ. 2013; Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signal. 25:961–969. DOI: 10.1016/j.cellsig.2013.01.007. PMID: 23333246. PMCID: PMC3595341.

97. Do DV, Ueda J, Messerschmidt DM, Lorthongpanich C, Zhou Y, Feng B, Guo G, Lin PJ, Hossain MZ, Zhang W, Moh A, Wu Q, Robson P, Ng HH, Poellinger L, Knowles BB, Solter D, Fu XY. 2013; A genetic and developmental pathway from STAT3 to the OCT4-NANOG circuit is essential for maintenance of ICM lineages in vivo. Genes Dev. 27:1378–1390. DOI: 10.1101/gad.221176.113. PMID: 23788624. PMCID: PMC3701193.

98. Zhao H, Guo Y, Li S, Han R, Ying J, Zhu H, Wang Y, Yin L, Han Y, Sun L, Wang Z, Lin Q, Bi X, Jiao Y, Jia H, Zhao J, Huang Z, Li Z, Zhou J, Song W, Meng K, Cai J. 2015; A novel anti-cancer agent Icaritin suppresses hepatocellular carcinoma initiation and malignant growth through the IL-6/Jak2/Stat3 pathway. Oncotarget. 6:31927–31943. DOI: 10.18632/oncotarget.5578. PMID: 26376676. PMCID: PMC4741651.

99. Yu X, Zhang F, Mao J, Lu Y, Li J, Ma W, Fan S, Zhang C, Li Q, Wang B, Song B, Li L. 2017; Protein tyrosine phosphatase receptor-type δ acts as a negative regulator suppressing breast cancer. Oncotarget. 8:98798–98811. DOI: 10.18632/oncotarget.22000. PMID: 29228728. PMCID: PMC5716768.
100. Chen Y, Shao Z, Jiang E, Zhou X, Wang L, Wang H, Luo X, Chen Q, Liu K, Shang Z. 2020; CCL21/CCR7 interaction promotes EMT and enhances the stemness of OSCC via a JAK2/STAT3 signaling pathway. J Cell Physiol. 235:5995–6009. DOI: 10.1002/jcp.29525. PMID: 32017846.

101. Liu HW, Lee PM, Bamodu OA, Su YK, Fong IH, Yeh CT, Chien MH, Kan IH, Lin CM. 2019; Enhanced hsa-miR-181d/p-STAT3 and hsa-miR-181d/p-STAT5A ratios mediate the anticancer effect of garcinol in STAT3/5A-addicted glio-blastoma. Cancers (Basel). 11:1888. DOI: 10.3390/cancers11121888. PMID: 31783691. PMCID: PMC6966688.

102. Wang L, Jiang Z, Huang D, Duan J, Huang C, Sullivan S, Vali K, Yin Y, Zhang M, Wegrzyn J, Tian XC, Tang Y. 2018; JAK/STAT3 regulated global gene expression dynamics during late-stage reprogramming process. BMC Genomics. 19:183. DOI: 10.1186/s12864-018-4507-2. PMID: 29510661. PMCID: PMC5840728.

103. Tang Y, Luo Y, Jiang Z, Ma Y, Lin CJ, Kim C, Carter MG, Amano T, Park J, Kish S, Tian XC. 2012; Jak/Stat3 signaling promotes somatic cell reprogramming by epigenetic regulation. Stem Cells. 30:2645–2656. DOI: 10.1002/stem.1225. PMID: 22968989.

104. Lin CS, Bamodu OA, Kuo KT, Huang CM, Liu SC, Wang CH, Tzeng YM, Chao TY, Yeh CT. 2018; Investigation of ovatodiolide, a macrocyclic diterpenoid, as a potential inhibitor of oral cancer stem-like cells properties via the inhibition of the JAK2/STAT3/JARID1B signal circuit. Phytomedicine. 46:93–103. DOI: 10.1016/j.phymed.2018.04.016. PMID: 30097127.

105. Su C. 2016; Survivin in survival of hepatocellular carcinoma. Cancer Lett. 379:184–190. DOI: 10.1016/j.canlet.2015.06.016. PMID: 26118774.

106. Wang G, Zhou H, Gu Z, Gao Q, Shen G. 2018; Oct4 promotes cancer cell proliferation and migration and leads to poor prognosis associated with the survivin/STAT3 pathway in hepatocellular carcinoma. Oncol Rep. 40:979–987. DOI: 10.3892/or.2018.6491. PMID: 29901157.

107. Martinez E, Vazquez N, Lopez A, Fanniel V, Sanchez L, Marks R, Hinojosa L, Cuello V, Cuevas M, Rodriguez A, Tomson C, Salinas A, Abad M, Holguin M, Garza N, Arenas A, Abraham K, Maldonado L, Rojas V, Basdeo A, Schuenzel E, Persans M, Innis-Whitehouse W, Keniry M. 2020; The PI3K pathway impacts stem gene expression in a set of glioblastoma cell lines. J Cancer Res Clin Oncol. 146:593–604. DOI: 10.1007/s00432-020-03133-w. PMID: 32030510.

108. Weidinger C, Krause K, Mueller K, Klagge A, Fuhrer D. 2011; FOXO3 is inhibited by oncogenic PI3K/Akt signaling but can be reactivated by the NSAID sulindac sulfide. J Clin Endocrinol Metab. 96:E1361–E1371. DOI: 10.1210/jc.2010-2453. PMID: 21752881.

109. Gomes AR, Zhao F, Lam EW. 2013; Role and regulation of the forkhead transcription factors FOXO3a and FOXM1 in carcinogenesis and drug resistance. Chin J Cancer. 32:365–370. DOI: 10.5732/cjc.012.10277. PMID: 23706767. PMCID: PMC3845605.

110. Bhummaphan N, Chanvorachote P. 2015; Gigantol suppresses cancer stem cell-like phenotypes in lung cancer cells. Evid Based Complement Alternat Med. 2015:836564. DOI: 10.1155/2015/836564. PMID: 26339272. PMCID: PMC4539074.

111. Chen B, Xue Z, Yang G, Shi B, Yang B, Yan Y, Wang X, Han D, Huang Y, Dong W. 2013; Akt-signal integration is involved in the differentiation of embryonal carcinoma cells. PLoS One. 8:e64877. DOI: 10.1371/journal.pone.0064877. PMID: 23762260. PMCID: PMC3675137.

112. Li X, Meng Y, Xie C, Zhu J, Wang X, Li Y, Geng S, Wu J, Zhong C, Li M. 2018; Diallyl Trisulfide inhibits breast cancer stem cells via suppression of Wnt/β-catenin pathway. J Cell Biochem. 119:4134–4141. DOI: 10.1002/jcb.26613. PMID: 29243835.

113. Chen SM, Lee MS, Chang CY, Lin SZ, Cheng EH, Liu YH, Pan HC, Lee HC, Su HL. 2015; Prerequisite OCT4 maintenance potentiates the neural induction of differentiating human embryonic stem cells and induced pluripotent stem cells. Cell Transplant. 24:829–844. DOI: 10.3727/096368913X675179. PMID: 24256943.

114. Guo Y, Li B, Yan X, Shen X, Ma J, Liu S, Zhang D. 2020; Bisphenol A and polychlorinated biphenyls enhance the cancer stem cell properties of human ovarian cancer cells by activating the WNT signaling pathway. Chemosphere. 246:125775. DOI: 10.1016/j.chemosphere.2019.125775. PMID: 31918092.

115. Simandi Z, Horvath A, Wright LC, Cuaranta-Monroy I, De Luca I, Karolyi K, Sauer S, Deleuze JF, Gudas LJ, Cowley SM, Nagy L. 2016; OCT4 acts as an integrator of pluripotency and signal-induced differentiation. Mol Cell. 63:647–661. DOI: 10.1016/j.molcel.2016.06.039. PMID: 27499297.

116. Davidson KC, Adams AM, Goodson JM, McDonald CE, Potter JC, Berndt JD, Biechele TL, Taylor RJ, Moon RT. 2012; Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc Natl Acad Sci U S A. 109:4485–4490. DOI: 10.1073/pnas.1118777109. PMID: 22392999. PMCID: PMC3311359.

117. Wang Y, Zhong Y, Hou T, Liao J, Zhang C, Sun C, Wang G. 2019; PM2.5 induces EMT and promotes CSC properties by activating Notch pathway in vivo and vitro. Ecotoxicol Environ Saf. 178:159–167. DOI: 10.1016/j.ecoenv.2019.03.086. PMID: 31002970.

118. Zhou ZC, Dong QG, Fu DL, Gong YY, Ni QX. 2013; Characteristics of Notch2+ pancreatic cancer stem-like cells and the relationship with centroacinar cells. Cell Biol Int. 37:805–811. DOI: 10.1002/cbin.10102. PMID: 23536545.

119. Jung N, Kwon HJ, Jung HJ. 2018; Downregulation of mitochondrial UQCRB inhibits cancer stem cell-like properties in glioblastoma. Int J Oncol. 52:241–251. DOI: 10.3892/ijo.2017.4191. PMCID: PMC5505016. PMID: 29115404.

120. Au HK, Chang JH, Wu YC, Kuo YC, Chen YH, Lee WC, Chang TS, Lan PC, Kuo HC, Lee KL, Lee MT, Tzeng CR, Huang YH. 2015; TGF-βI regulates cell migration through pluripotent transcription factor OCT4 in endometriosis. PLoS One. 10:e0145256. DOI: 10.1371/journal.pone.0145256. PMID: 26675296. PMCID: PMC4682958.
121. Yuan F, Zhou W, Zou C, Zhang Z, Hu H, Dai Z, Zhang Y. 2010; Expression of Oct4 in HCC and modulation to wnt/β-catenin and TGF-β signal pathways. Mol Cell Biochem. 343:155–162. DOI: 10.1007/s11010-010-0509-3. PMID: 20549546.

122. Liang K, Zhou G, Zhang Q, Li J, Zhang C. 2014; Expression of hippo pathway in colorectal cancer. Saudi J Gastroenterol. 20:188–194. DOI: 10.4103/1319-3767.133025. PMID: 24976283. PMCID: PMC4067916.

123. Haghighi F, Dahlmann J, Nakhaei-Rad S, Lang A, Kutschka I, Zenker M, Kensah G, Piekorz RP, Ahmadian MR. 2018; bFGF-mediated pluripotency maintenance in human induced pluripotent stem cells is associated with NRAS-MAPK signaling. Cell Commun Signal. 16:96. DOI: 10.1186/s12964-018-0307-1. PMID: 30518391. PMCID: PMC6282345.

124. Jung JS, Jee MK, Cho HT, Choi JI, Im YB, Kwon OH, Kang SK. 2013; MBD6 is a direct target of Oct4 and controls the stemness and differentiation of adipose tissue-derived stem cells. Cell Mol Life Sci. 70:711–728. DOI: 10.1007/s00018-012-1157-4. PMID: 23052207.

125. Jang JH, Jung JS, Im YB, Kang KS, Choi JI, Kang SK. 2012; Crucial role of nuclear Ago2 for hUCB-MSCs differentiation and self-renewal via stemness control. Antioxid Redox Signal. 16:95–111. DOI: 10.1089/ars.2011.3975. PMID: 21902595.

126. Bie Q, Zhang B, Sun C, Ji X, Barnie PA, Qi C, Peng J, Zhang D, Zheng D, Su Z, Wang S, Xu H. 2017; IL-17B activated mesenchymal stem cells enhance proliferation and migration of gastric cancer cells. Oncotarget. 8:18914–18923. DOI: 10.18632/oncotarget.14835. PMID: 28145881. PMCID: PMC5386657.

127. Wang D, Xiang T, Zhao Z, Lin K, Yin P, Jiang L, Liang Z, Zhu B. 2016; Autocrine interleukin-23 promotes self-renewal of CD133+ ovarian cancer stem-like cells. Oncotarget. 7:76006–76020. DOI: 10.18632/oncotarget.12579. PMID: 27738346. PMCID: PMC5342794.

128. Kim KW, Kim JY, Qiao J, Clark RA, Powers CM, Correa H, Chung DH. 2019; Dual-Targeting AKT2 and ERK in cancer stem-like cells in neuroblastoma. Oncotarget. 10:5645–5659. DOI: 10.18632/oncotarget.27210. PMID: 31608140. PMCID: PMC6771463.

129. Tang J, Li L, Huang W, Sui C, Yang Y, Lin X, Hou G, Chen X, Fu J, Yuan S, Li S, Wen W, Tang S, Cao D, Wu M, Chen L, Wang H. 2015; MiR-429 increases the metastatic capability of HCC via regulating classic Wnt pathway rather than epithelial-mesenchymal transition. Cancer Lett. 364:33–43. DOI: 10.1016/j.canlet.2015.04.023. PMID: 25931210.

130. Li L, Tang J, Zhang B, Yang W, LiuGao M, Wang R, Tan Y, Fan J, Chang Y, Fu J, Jiang F, Chen C, Yang Y, Gu J, Wu D, Guo L, Cao D, Li H, Cao G, Wu M, Zhang MQ, Chen L, Wang H. 2015; Epigenetic modification of MiR-429 promotes liver tumour-initiating cell properties by targeting Rb binding protein 4. Gut. 64:156–167. DOI: 10.1136/gutjnl-2013-305715. PMID: 24572141.

131. Shigeishi H, Biddle A, Gammon L, Emich H, Rodini CO, Gemenetzidis E, Fazil B, Sugiyama M, Kamata N, Mackenzie IC. 2013; Maintenance of stem cell self-renewal in head and neck cancers requires actions of GSK3β influenced by CD44 and RHAMM. Stem Cells. 31:2073–2083. DOI: 10.1002/stem.1418. PMID: 23649588.

132. Hu J, Qin K, Zhang Y, Gong J, Li N, Lv D, Xiang R, Tan X. 2011; Downregulation of transcription factor Oct4 induces an epithelial-to-mesenchymal transition via enhancement of Ca2+ influx in breast cancer cells. Biochem Biophys Res Commun. 411:786–791. DOI: 10.1016/j.bbrc.2011.07.025. PMID: 21798248.

133. Xun J, Wang D, Shen L, Gong J, Gao R, Du L, Chang A, Song X, Xiang R, Tan X. 2017; JMJD3 suppresses stem cell-like characteristics in breast cancer cells by downregulation of Oct4 independently of its demethylase activity. Oncotarget. 8:21918–21929. DOI: 10.18632/oncotarget.15747. PMID: 28423536. PMCID: PMC5400634.

134. Chen T, Liu K, Xu J, Zhan T, Liu M, Li L, Yang Z, Yuan S, Zou W, Lin G, Carson DA, Wu CCN, Wang X. 2020; Synthetic and immunological studies on the OCT4 immunodominant motif antigen-based anti-cancer vaccine. Cancer Biol Med. 17:132–141. DOI: 10.20892/j.issn.2095-3941.2019.0224. PMID: 32296581. PMCID: PMC7142840.

135. Yan Y, Liu F, Han L, Zhao L, Chen J, Olopade OI, He M, Wei M. 2018; HIF-2α promotes conversion to a stem cell phenotype and induces chemoresistance in breast cancer cells by activating Wnt and Notch pathways. J Exp Clin Cancer Res. 37:256. DOI: 10.1186/s13046-018-0925-x. PMID: 30340507. PMCID: PMC6194720.
136. Asadi MH, Khalifeh K, Mowla SJ. 2016; OCT4 spliced variants are highly expressed in brain cancer tissues and inhibition of OCT4B1 causes G2/M arrest in brain cancer cells. J Neurooncol. 130:455–463. DOI: 10.1007/s11060-016-2255-1. PMID: 27585657.

Fig. 1
Epigenetic modification related to OCT4. This figure tries to explain the role of epigenetic modification in different periods of OCT4 expression. Met: methylation, P: phosphorylation, Ac: acetylation, Ub: ubiquitination, SUMO: SUMOylation, O-GlcNAc: monosaccharide O-linked β-N-acetylglucosamine.
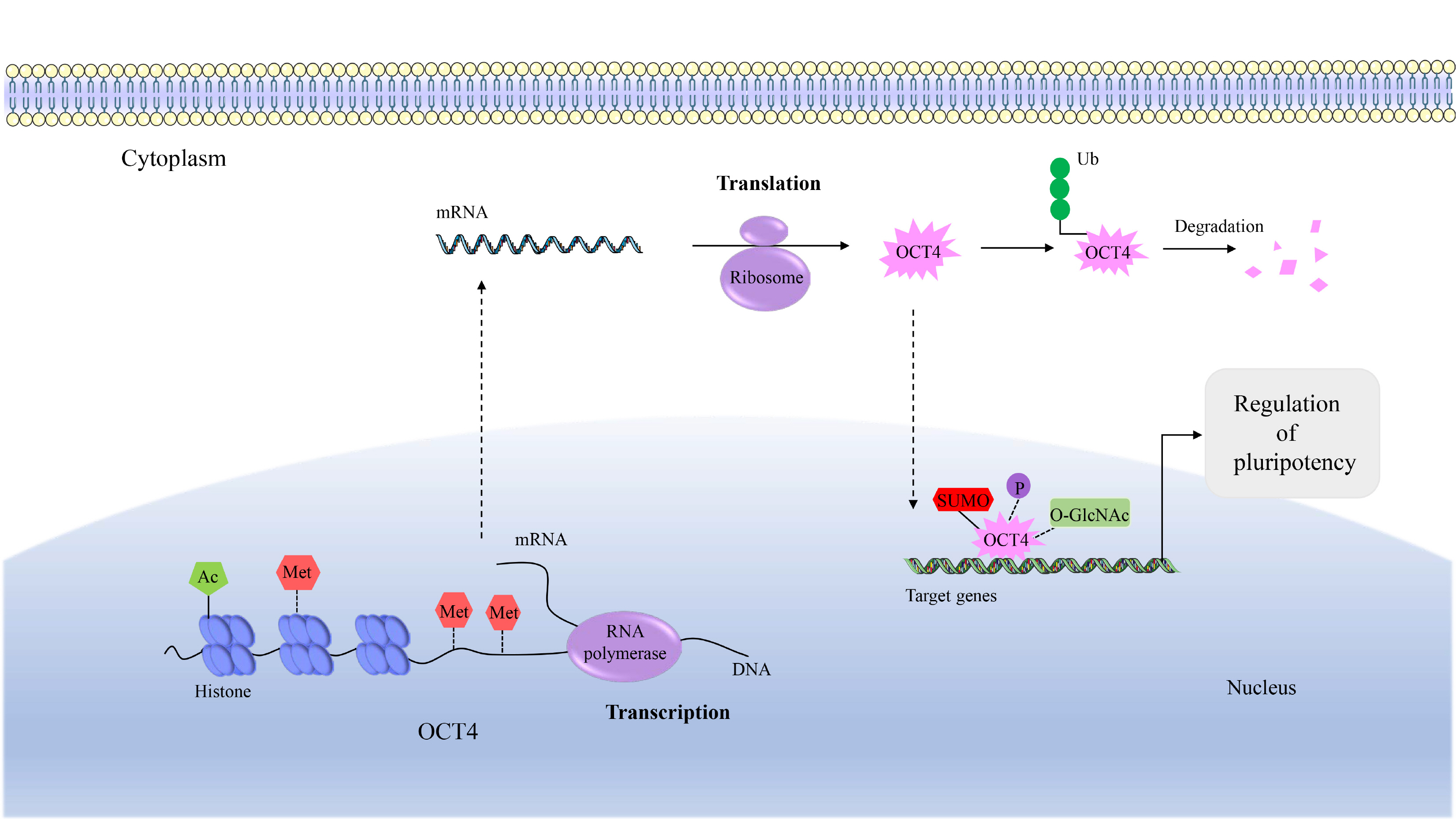
Fig. 2
Several OCT4-related signaling pathways that participate in regulating CSCs. This figure shows several OCT4-related signaling pathways that participate in regulating CSCs.
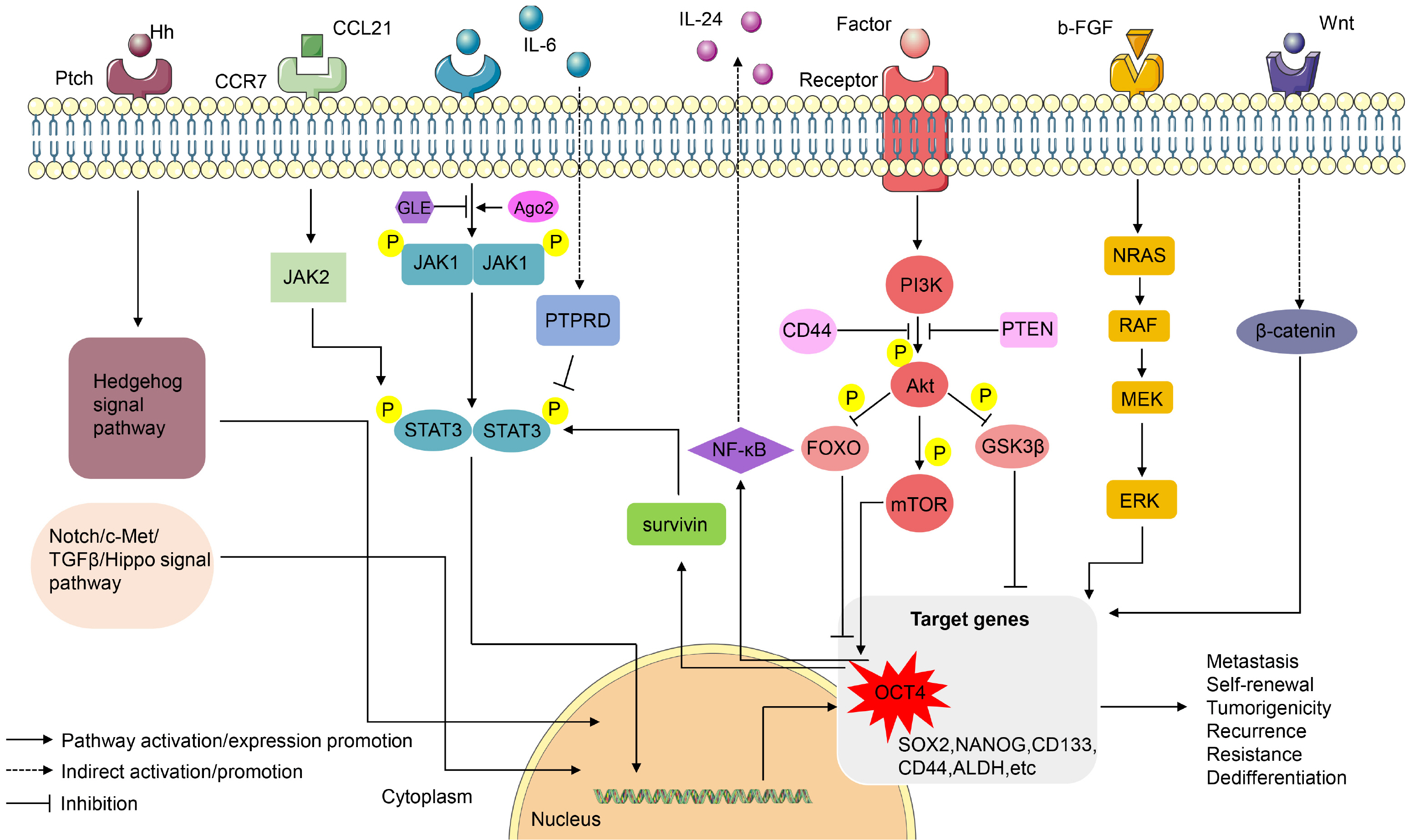
Table 1
The role and mechanism of ncRNA interacting with OCT4 in CSCs
Table 2
The role of OCT4-related complexes in cancer stem cells




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download