1. Foster CA. Optimal management of Meniere’s disease. Ther Clin Risk Manag. 2015; Feb. 11:301–7.
2. Nevoux J, Barbara M, Dornhoffer J, Gibson W, Kitahara T, Darrouzet V. International consensus (ICON) on treatment of Meniere’s disease. Eur Ann Otorhinolaryngol Head Neck Dis. 2018; Feb. 135(1S):S29–32.
3. Kitahara M, Takeda T, Yazawa Y, Matsubara H, Kitano H. Treatment of Meniere’s disease with isosorbide. ORL J Otorhinolaryngol Relat Spec. 1982; 44(4):232–8.
4. Juhn SK, Prado S, Pearce J. Osmolality changes in perilymph after systemic administration of glycerin. Arch Otolaryngol. 1976; Nov. 102(11):683–5.

5. Kakigi A, Takeda T, Saito H, Kataoka H. Effect of isosorbide on hearing loss due to endolymphatic hydrops. Acta Otolaryngol Suppl. 1995; 519:223–6.

6. Takeda T, Takeuchi S, Saito H. Effect of glycerol on pressure difference between perilymph and endolymph. Acta Otolaryngol. 1990; Jul–Aug. 110(1–2):68–72.

7. Kakigi A, Takeda S, Takeda T, Sawada S, Azuma H, Higashiyama K, et al. Time course of dehydrating effects of isosorbide on experimentally induced endolymphatic hydrops in guinea pigs. ORL J Otorhinolaryngol Relat Spec. 2004; 66(6):291–6.

8. Padoan S. Oral versus i.v. administration of the glycerol test: side-effects and usefulness. Acta Otolaryngol. 2003; May. 123(4):482–7.

9. Matsubara H, Kitahara M, Takeda T, Yazawa Y. Rebound phenomenon in glycerol test. Acta Otolaryngol Suppl. 1984; 419:115–22.

10. Larsen HC, Angelborg C, Klockoff I, Stahle J. The effect of isosorbide and urea on hearing in patients with Meniere’s disease. Acta Otolaryngol (Stockh). 1984; 412:113–4.
11. Yamazaki T, Imoto T, Hayashi N, Watanabe S, Kozaki H, Abe T. Meniere’s disease and isosorbide as an oral hyperosmotic agent. Arch Otorhinolaryngol. 1982; 234(1):97–104.
12. Nozawa I, Nakayama H, Hashimoto K, Imamura S, Hisamatu K, Murakami Y. Efficacy of long-term administration of isosorbide for Meniere’s disease. ORL J Otorhinolaryngol Relat Spec. 1995; May–Jun. 57(3):135–40.
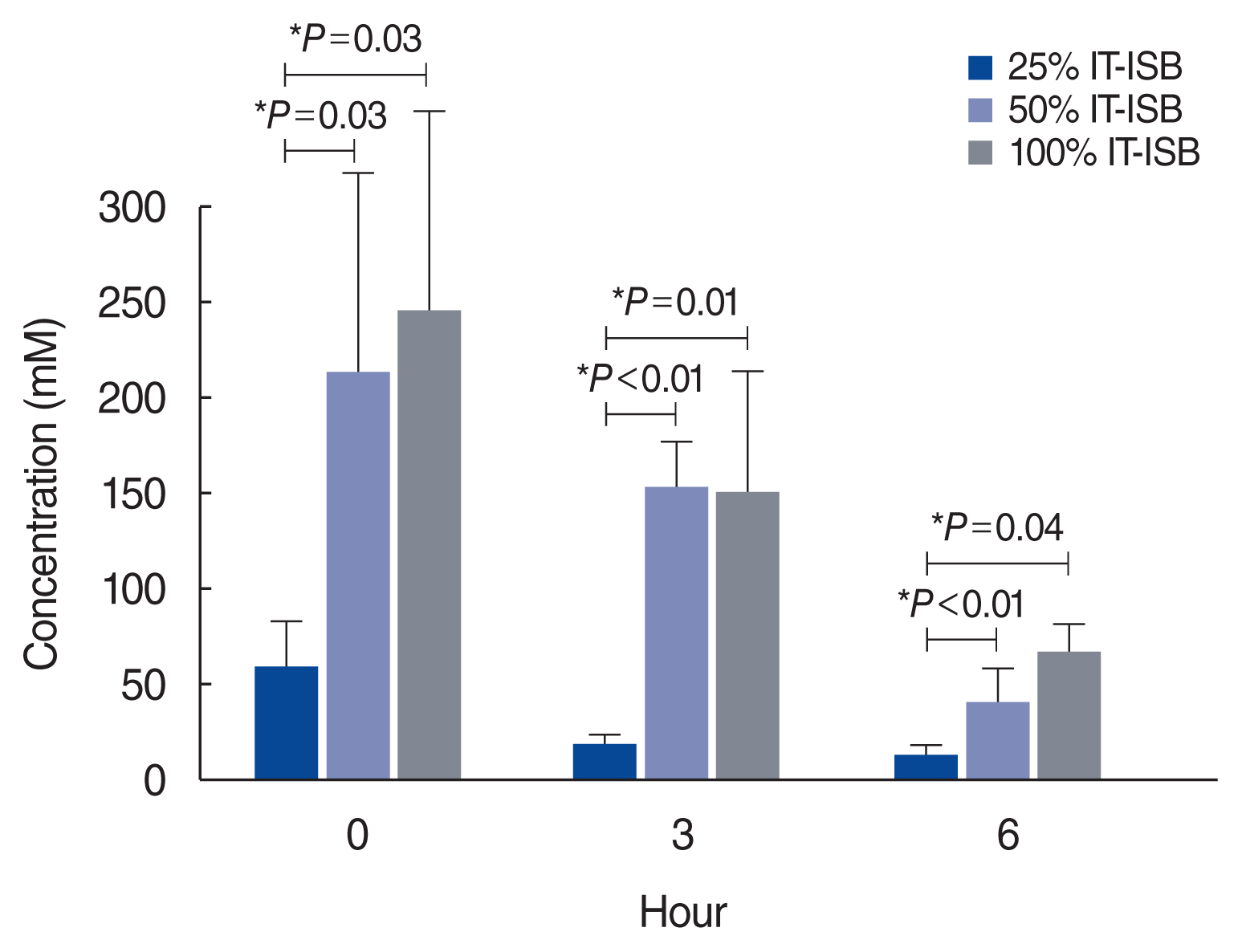
13. Kim M, Do KH, Kim KS. Isosorbide concentration in perilymph of the guinea pig after oral administration versus that after round window perfusion. Clin Exp Otorhinolaryngol. 2014; Dec. 7(4):281–5.

14. Kakigi A, Takeda T, Sawada S, Taguchi D. Antidiuretic hormone and osmolality in isosorbide therapy and glycerol test. ORL J Otorhinolaryngol Relat Spec. 2006; Aug. 68(5):279–82.

15. Klockhoff I. Diagnosis of Meniere’s disease. Arch Otorhinolaryngol. 1976; Sep. 212(4):309–14.
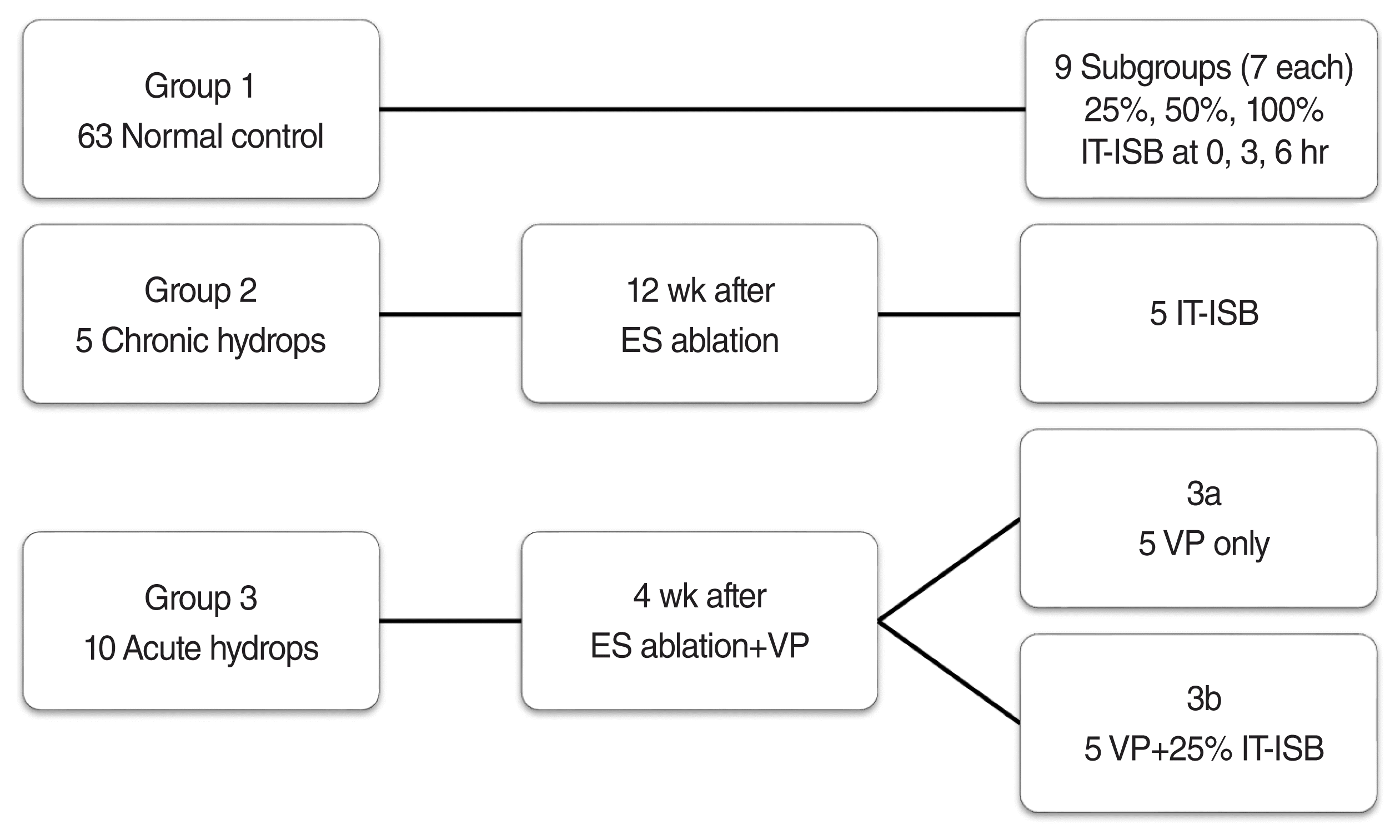
16. Egami N, Kakigi A, Sakamoto T, Takeda T, Hyodo M, Yamasoba T. Morphological and functional changes in a new animal model of Meniere’s disease. Lab Invest. 2013; Sep. 93(9):1001–11.
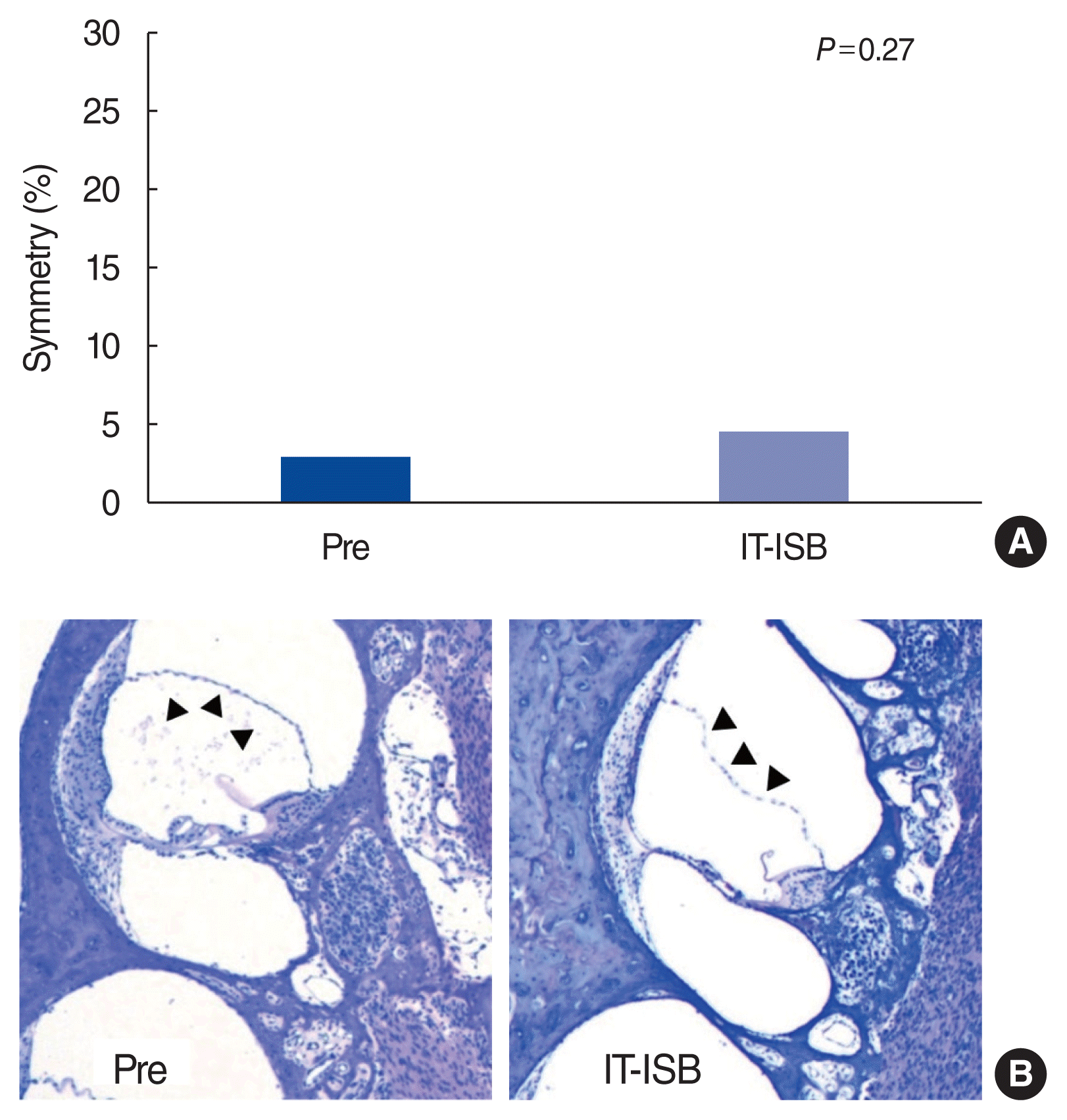
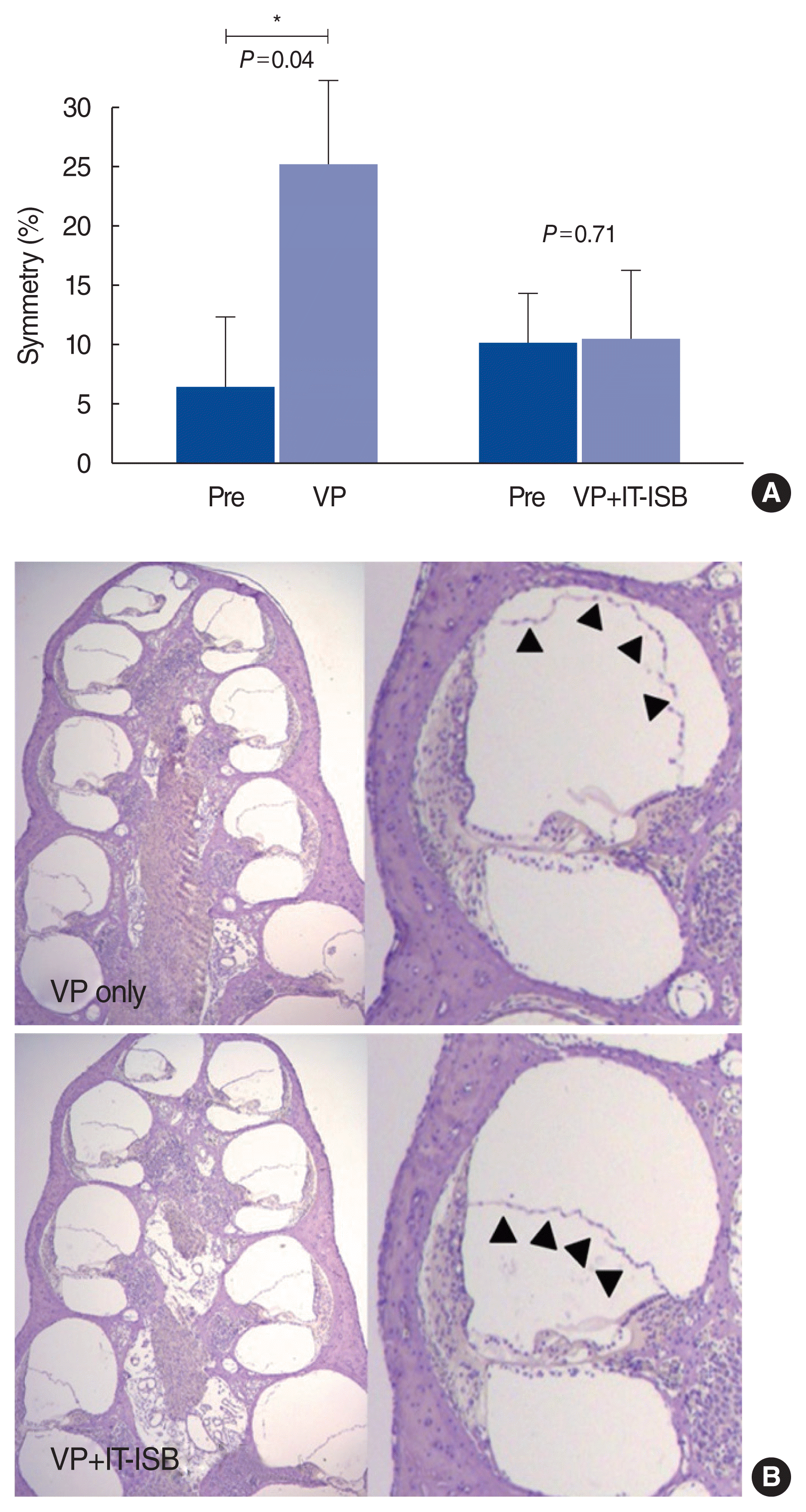
17. Kim M, Kim KS. Vestibular function change in a vasopressin-induced hydrops model. Otol Neurotol. 2017; Dec. 38(10):e495–500.

18. Salt AN, Plontke SK. Endolymphatic hydrops: pathophysiology and experimental models. Otolaryngol Clin North Am. 2010; Oct. 43(5):971–83.

19. Lopez-Escamez JA, Carey J, Chung WH, Goebel JA, Magnusson M, Mandala M, et al. Diagnostic criteria for Meniere’s disease. J Vestib Res. 2015; 25(1):1–7.
20. Sajjadi H, Paparella MM. Meniere’s disease. Lancet. 2008; Aug. 372(9636):406–14.

21. Shigeno K, Egami T, Sasano T. Experimental study of nystagmus induced by injecting various solutions into the middle ear cavity. Acta Otolaryngol. 1989; Jul–Aug. 108(1–2):31–7.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download