Abstract
Background
Hospital-acquired pneumonia (HAP) is a common complication after abdominal surgery. The aim of this study was to evaluate the role of procalcitonin (PCT) and C-reactive protein (CRP) as early biomarkers for the diagnosis of postoperative HAP after abdominal surgery.
Methods
This study was conducted on 100 patients undergoing abdominal surgery. White blood cell counts, highest body temperature, and serum levels of CRP and PCT were recorded preoperatively and daily postoperatively until postoperative day (POD) 5. Chest radiography was performed preoperatively and daily postoperatively until POD 5.
Results
HAP was diagnosed in 14% of patients. Regarding the biomarkers studied after POD 1, CRP and PCT were significantly higher in patients with HAP than in those without HAP (P < 0.05). On POD 2, PCT had higher sensitivity and specificity (84% and 72%, respectively) than those for CPR (70% and 60%, respectively). The cut-off value of PCT on POD 2 was 1.4 ng/ml. On POD 3, 4, and 5, the sensitivity and specificity of PCT and CRP were not significantly different.
Pulmonary complications after surgery are common and contribute to the risk of morbidity and mortality of surgery with a longer hospital stay [1]. Early diagnosis of hospital-acquired pneumonia (HAP) can limit the morbidity and mortality of such conditions. Various biomarkers are evaluated for early identification of this infection as C-reactive protein (CRP) and procalcitonin (PCT). CRP is one member of the acute-phase proteins used as a biomarker for the detection of inflammation, tissue damage, and infection [2]. Thus, it is not a specific marker for infection. However, several studies have shown that CRP is a useful predictor for postoperative infectious complications [34].
The other biomarker, PCT, a precursor of calcitonin hormone, is synthesized by C cells of the thyroid gland [5]. PCT is known to be increased in bacterial infections, making it a potential target for the early identification of various infections, especially sepsis [67]. Moreover, it can be used for diagnosis of bacterial pneumonia, especially associated with a ventilator [8]. Burns, severe trauma, and surgery are also known to increase PCT levels, even in the absence of infection [91011].
The aim of the present study was to evaluate the use of PCT and CRP as biomarkers for the early diagnosis of postoperative HAP and examine perioperative risk factors for HAP after abdominal surgery.
A prospective observational study was conducted on 100 patients between August 2015 and July 2016. The study protocol was approved by the Ethics Committee (MFM-IRB; R/16.03.47) and patients provided written informed consent. Patients had undergone elective abdominal surgery for upper-gastrointestinal, hepato-pancreatico-biliary, or colorectal resections.
Patients with the following illnesses were excluded from the study; patients who received immunosuppressants or long-term corticosteroid therapy, those with a coexisting extrapulmonary infection prior to or following surgery, those with mechanical ventilation at the time of preoperative assessment, pregnant women, and those with chronic renal insufficiency or liver dysfunction.
Metronidazole (500 mg i.v.) and cefamandole (2,000 mg i.v.), prophylactic antibiotics, were administered 45 min before surgery and were continued for up to 2 days after surgery in all patients.
Recorded data included age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) status, smoking history, chronic obstructive pulmonary disease (COPD), asthma and other co-morbidity history, type and duration of surgery, length of intensive care unit (ICU), and hospital stay.
White blood cell count (WBC), highest measured body temperature, and serum levels of CRP and PCT were recorded preoperatively and daily postoperatively until postoperative day (POD) 5.
Chest radiography was performed preoperatively and daily until POD 5. Postoperatively, patients were evaluated daily for symptoms of pneumonia. Patients with sustained elevation of PCT and/or CRP were investigated according to clinical presentation for the diagnosis and exclusion of extrapulmonary infective complications. When pneumonia was diagnosed, the patients received 1.2 g amoxicillin and clavulanate (Augmentin, Medical Union Pharmaceuticals-Egypt; under license from the GlaxoSmithKline Group of Companies) every 8 h as empirical antibiotic treatment until the results of sputum culture were received. At this point, the treatment was adapted to the detected pathogen(s).
Diagnosis of HAP was based on the appearance of new pulmonary infiltrates on chest X-ray, with at least two of the following features: fever with a body temperature of > 38℃, white blood cell count of > 11,000 or < 3,000 /mm3, or the presence of purulent sputum [12]. Full microbiological culture of sputum samples was performed for identification of bacterial isolates. Culture was considered positive if ≥ 106 colony forming units/ml were detected. Blood samples were separated and kept frozen at −20℃ for determination of CRP and PCT levels using enzyme-linked immunosorbent assay (ELISA).
The primary goal of the present study was assessment of the role of PCT in early prediction of HAP after major abdominal surgery and comparing its diagnostic accuracy to that of CRP based on analysis of serial daily postoperative serum PCT and serum CRP from POD 1 to 5. The secondary goal was identification of the perioperative risk factors for HAP after abdominal surgery.
This assay uses monoclonal antibodies specific for PCT coated on a plate. The samples and standards are pipetted to the wells to bind to antibodies. After washing, biotinylated anti-human PCT antibody is added. A second wash is then performed and antibody labeled with streptavidin is added. The wells are again washed and a Tetramethylbenzidine substrate solution is added to the wells, the color of which develops in proportion to the amount of bound PCT. The stop solution changes the color from blue to yellow, and the intensity of the color is measured at 450 nm.
This assay uses specific monoclonal antibodies for CRP in solid immunoassay plates.
Calculation of the sample size was based on the diagnostic accuracy of PCT and CRP to predict postoperative HAP. Depending on the results of previous reports [13], 97 patients would be needed to detect a 17% difference in the diagnostic accuracy between PCT and CRP at an α error of 0.05 and with a study power of 80%. Data were analyzed using SPSS statistics software ver.16 (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was performed to verify the assumption of normality. Results are expressed as the mean ± SD or median with interquartile range for continuous variables, while categorical variables are expressed as number (n) or percentage (%). Categorical data were compared between patients with and without HAP based on the chi-square test (sex, ASA status, smoking status, and surgical incision) or Fisher's exact test (co-morbidities: COPD and asthma). Continuous data were compared using Student's t-test or the Mann-Whitney U test (CRP and PCT kinetics). Intragroup changes in CRP and PCT kinetics were analyzed using the Wilcoxon signed-rank test. The receiver operating characteristic (ROC) curve and the value of the area under the curve (AUC) were used to analyze the accuracy of PCT and CRP as predictors for early diagnosis of HAP at POD 2, 3, 4, and 5. AUC provided a direct measure of the diagnostic accuracy of the test. AUC was compared using nonparametric method that described by Hanley and McNeil [14]. The multivariate logistic regression model was constructed to determine the risk factors for the development of postoperative HAP. Univariate logistic regression was performed to study the perioperative independent factors associated with the development of HAP. Then multivariate logistic regression method was performed for variables that were significant in the univariate logistic regression (P < 0.05). Preoperative factors (age, sex, BMI, smoking status, and history of COPD or asthma) and intraoperative factors (site of surgical incision and duration of surgery) were included in the logistic regression model. A two-tailed P value < 0.05 was considered significant.
A total of 100 patients undergoing major abdominal surgery were prospectively enrolled in the study. Postoperative HAP was diagnosed in 14 patients (14%). In total, 3, 7, and 4 patients were diagnosed at POD 4, 5, and 6, respectively, with a median value of POD 5. A comparison of demographic and perioperative data between patients with postoperative HAP and those without is shown in Table 1. Prolonged ICU stay (7 days) and hospital stay (13 days) were significantly associated with the development of postoperative HAP (P < 0.001).
Table 2 shows a summary of the univariate and multivariate logistic regression analyses of perioperative independent factors associated with the development of postoperative HAP. The studied significant risk factors were age ≥ 60 years, smoking ≥ 40 pack-years, upper or upper/lower abdominal incision, and duration of surgery ≥ 3 h (P = 0.001, 0.014, 0.011, and 0.031, respectively).
The most common bacterial pathogens isolated in the present study were Staphylococcus aureus (35.7%), followed by Pseudomonas aeruginosa (28.6%), E. coli (21.4%), and Klebsiella species (14.3%).
Comparing patients with and without postoperative HAP, no significant differences were detected in WBC count (cells/mm3) either preoperatively or throughout the first 4 days postoperatively (Table 3).
CRP levels in patients with and without postoperative HAP showed insignificant differences in preoperative values (P = 0.873). The levels increased significantly on POD 1 in patients with and without postoperative HAP; the median values were 105 mg/L (interquartile range 80.0–122.5) and 92 mg/L (78.8–110.0), with peaks on POD 2 at 165 mg/L (133.8–186.3) and 135 mg/L (112.8–154.0), respectively. From POD 3, CRP levels decreased progressively in patients without postoperative HAP until reaching a value of 57 mg/L (48.0–67.5) on POD 5. In patients with postoperative HAP, CRP levels remained increased from POD 2. Comparison of the CRP levels between patients with and without HAP revealed significant differences on POD 2, 3, 4, and 5 (Fig. 1).
Preoperative PCT levels in patients with and without postoperative HAP were not significantly different (P = 0.258). On POD 1, PCT increased significantly in patients with and without postoperative HAP, in whom it was 1.04 ng/ml (0.78–1.40) and 0.8 ng/ml (0.69–1.09), respectively, with no significant difference between them (P = 0.153). In patients without postoperative HAP, PCT level significantly decreased from POD 2 to 0.22 ng/ml (0.18–0.30) on POD 5. In patients with postoperative HAP, it increased on POD 2 to 1.52 ng/ml (1.17–1.91) and remained elevated thereafter (Fig. 2).
At the time of diagnosis of postoperative HAP, the median CRP levels were 150 mg/L and 148.5 mg/L on POD 4 and POD 5, while the median PCT levels were 1.5 and 1.53 ng/ml, respectively. ROC curves for the postoperative levels of PCT and CRP are shown in Figs. 3 and 4. ROC curve analysis on POD 2 and 3 was used to assess the predictive value of CRP and PCT levels for the early diagnosis of postoperative HAP, as the median day for the diagnosis of postoperative HAP in our study was POD 5 (POD 4–6). On POD 2, AUC of PCT was 0.844 (95% CI: 0.765–0.895) with a sensitivity of 84%, specificity of 72%, and a cut-off value of 1.4 ng/ml, while AUC of CRP was 0.716 (95% CI: 0.658–0.874) with a sensitivity of 70%, specificity of 60%, and a cut-off value of 155 mg/L. On the same day, the diagnostic accuracy of PCT was significantly better than that of CRP, AUC of PCT was significantly higher than that of CRP (P = 0.018). The diagnostic accuracy of PCT for postoperative HAP was not significantly higher than that of CRP on POD 3, 4, and 5 (P = 0.584, 0.453, and 0.593, respectively). On POD 3, AUC values of PCT and CRP were 0.895 (95% CI: 0.812–0.945, sensitivity 88%, and specificity 75%) and 0.864 (95% CI: 0.785–0.897, sensitivity 84%, and specificity 73%), respectively (P = 0.584). The cut-off values of PCT and CRP on POD 3 were 1.4 ng/ml and 145 mg/L, respectively.
In our study, postoperative HAP was reported in 14% of patients. Postoperative pneumonia after abdominal surgery is reported to range from 9 to 40% [151617].
CRP is an acute-phase protein released from the liver after stimulation. Secretion begins 4–6 h after stimulation and peaks around 48 h [2]. PCT can be detected in the circulation within 4 h after an adequate stimulus, with peak values at 8 h [18].
The results of our study showed that the diagnostic accuracy of PCT was significantly better than that of CRP on POD 2 as comparing the AUC. On POD 3, 4, and 5, there was no significant difference between the diagnostic accuracy of PCT and CRP. In our study, the median day for the diagnosis of postoperative HAP was POD 5 (range of 4–6 days). We considered the analysis of AUC for CRP and PCT on POD 2 and 3 as a predictor of the development of postoperative HAP. WBC count and body temperature on POD 2 and 3 were not significantly different between patients with and without postoperative HAP.
The results of our study showed that CRP increased postoperatively and reached its peak in patients with and without postoperative HAP on POD 2. After POD 2, CRP progressively decreased in patients without postoperative HAP, while it remained elevated in patients with postoperative HAP. On POD 3, AUC of CRP was 0.864 with sensitivity of 84% and specificity 73%. The cut-off value for CRP was 145 mg/L on POD 3. CRP has been proved to detect postoperative septic complications, especially if measured in a serial manner before and after abdominal or thoracic surgery [34]. Welsch et al. [19] in their study on 383 patients with cancer rectum undergoing rectal resection with primary anastomosis, they investigated the role of CRP in the prediction of postoperative infectious complications. They concluded that persistent elevation of CRP above 140 mg/L on PODs 3–4 is considered a good predictive marker for postoperative infective complications. Korner et al. [20] concluded that the persistent elevation of CRP after POD 3 with a cut-off value of 190 mg/L should be investigated for intra-abdominal infection.
The results of our research showed that PCT increased in patients with and without postoperative HAP on POD 1. After POD 1, PCT progressively decreased in patients without postoperative HAP, while it was elevated in patients with postoperative HAP. AUC of PCT was 0.844 with sensitivity of 84% and specificity of 72% on POD 2, and it was 0.895 with sensitivity of 88% and specificity of 75% on POD 3, with cut-off values of 1.4 ng/ml on both days.
PCT is produced at higher levels especially after gastrointestinal surgery. These elevated levels after abdominal surgery could be attributed to the transient bacterial translocation from the gastrointestinal tract due to malperfusion of the gut [11]. Oberhofer et al. [21] evaluated and compared the roles of perioperative CRP and PCT for the early detection of infective complications after colorectal surgery. They documented that the CRP level on POD 3 and PCT level on POD 2 had similar predictive values for the development of infectious complications, with the best cut-off values of 99.0 mg/L for CRP and 1.34 µg/L for PCT. The best diagnostic accuracy of postoperative CRP and PCT was obtained on POD 5, as the median day of clinical diagnosis of postoperative infections was POD 7 (range 5–14 days). Mokart et al. [22] explored the value of changes in serum levels of interleukin 6, PCT, and CRP in the prediction of septic complications in 50 patients undergoing major surgery for cancer. They concluded that PCT and IL-6 are useful early markers of subsequent postoperative sepsis in patients undergoing major surgery for cancer. The cut-off value of PCT on POD 1 in their study was 1.1 ng/ml, with sensitivity of 81% and specificity of 72%. Previous studies reported that PCT is an accurate marker for the early diagnosis of postoperative infective complication [56721] and ventilator-associated pneumonia [8]. On the other hand, some studies concluded that PCT had no added benefit in the prediction of postoperative infection [23]. Several studies have compared PCT and CRP for the diagnosis of postoperative infectious complications [52124], and PCT is documented to be more sensitive than CRP for the early detection of postoperative septic complications after major surgery in some previous studies [2225]. However, CRP was proven to be more sensitive than PCT in other studies [26].
The determination of risk factors associated with the development of pneumonia in surgical patients can be used to decrease the incidence of this complication. In the present study, significant risk factors for the development of postoperative HAP were age ≥ 60 years, smoking ≥ 40 pack-years, upper abdominal incision and duration of surgery ≥ 3 h. Several studies have demonstrated that age > 55 [2728], upper or upper/lower abdominal incision [27], smoking history [29], and duration of surgery more than 2.5 hours [28] correlated with postoperative pulmonary complications.
In conclusion, PCT and CRP are accurate biomarkers for the early prediction of postoperative HAP after abdominal surgery. The diagnostic ability of PCT was significantly better than that of CRP on POD 2. After POD 2, the diagnostic ability was not significantly different between both biomarkers. Persistent elevation of PCT to a level higher than 1.4 ng/ml on POD 2 and 3, and CRP levels higher than 145 mg/L on POD 3 after abdominal surgery, may be suggestive of postoperative infectious complications. However, further studies are required to validate our finding.
References
1. Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006; 144:581–595. PMID: 16618956.

2. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003; 111:1805–1812. PMID: 12813013.

3. MacKay GJ, Molloy RG, O'Dwyer PJ. C-reactive protein as a predictor of postoperative infective complications following elective colorectal resection. Colorectal Dis. 2011; 13:583–587. PMID: 20163424.

4. Dutta S, Fullarton GM, Forshaw MJ, Horgan PG, McMillan DC. Persistent elevation of C-reactive protein following esophagogastric cancer resection as a predictor of postoperative surgical site infectious complications. World J Surg. 2011; 35:1017–1025. PMID: 21350898.

5. Garcia-Granero A, Frasson M, Flor-Lorente B, Blanco F, Puga R, Carratalá A, et al. Procalcitonin and C-reactive protein as early predictors of anastomotic leak in colorectal surgery: a prospective observational study. Dis Colon Rectum. 2013; 56:475–483. PMID: 23478615.
6. Falcoz PE, Laluc F, Toubin MM, Puyraveau M, Clement F, Mercier M, et al. Usefulness of procalcitonin in the early detection of infection after thoracic surgery. Eur J Cardiothorac Surg. 2005; 27:1074–1078. PMID: 15896620.

7. Takakura Y, Hinoi T, Egi H, Shimomura M, Adachi T, Saito Y, et al. Procalcitonin as a predictive marker for surgical site infection in elective colorectal cancer surgery. Langenbecks Arch Surg. 2013; 398:833–839. PMID: 23784676.

8. Duflo F, Debon R, Monneret G, Bienvenu J, Chassard D, Allaouchiche B. Alveolar and serum procalcitonin: diagnostic and prognostic value in ventilator-associated pneumonia. Anesthesiology. 2002; 96:74–79. PMID: 11753005.
9. Carsin H, Assicot M, Feger F, Roy O, Pennacino I, Le Bever H, et al. Evolution and significance of circulating procalcitonin levels compared with IL-6, TNF alpha and endotoxin levels early after thermal injury. Burns. 1997; 23:218–224. PMID: 9232281.
10. Mimoz O, Benoist JF, Edouard AR, Assicot M, Bohuon C, Samii K. Procalcitonin and C-reactive protein during the early posttraumatic systemic inflammatory response syndrome. Intensive Care Med. 1998; 24:185–188. PMID: 9539079.

11. Meisner M, Tschaikowsky K, Hutzler A, Schick C, Schüttler J. Postoperative plasma concentrations of procalcitonin after different types of surgery. Intensive Care Med. 1998; 24:680–684. PMID: 9722037.

12. American Thoracic Society. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005; 171:388–416. PMID: 15699079.
13. Jebali MA, Hausfater P, Abbes Z, Aouni Z, Riou B, Ferjani M. Assessment of the accuracy of procalcitonin to diagnose postoperative infection after cardiac surgery. Anesthesiology. 2007; 107:232–238. PMID: 17667566.

14. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983; 148:839–843. PMID: 6878708.

15. Calligaro KD, Azurin DJ, Dougherty MJ, Dandora R, Bajgier SM, Simper S, et al. Pulmonary risk factors of elective abdominal aortic surgery. J Vasc Surg. 1993; 18:914–920. PMID: 8264047.

16. Dilworth JP, Warley AR, Dawe C, White RJ. The effect of nebulized salbutamol therapy on the incidence of postoperative chest infection in high risk patients. Respir Med. 1994; 88:665–668. PMID: 7809438.

17. Ephgrave KS, Kleiman-Wexler R, Pfaller M, Booth B, Werkmeister L, Young S. Postoperative pneumonia: a prospective study of risk factors and morbidity. Surgery. 1993; 114:815–819. PMID: 8211699.
18. Nijsten MW, Olinga P, The TH, de Vries EG, Koops HS, Groothuis GM, et al. Procalcitonin behaves as a fast responding acute phase protein in vivo and in vitro. Crit Care Med. 2000; 28:458–461. PMID: 10708183.

19. Welsch T, Müller SA, Ulrich A, Kischlat A, Hinz U, Kienle P, et al. C-reactive protein as early predictor for infectious postoperative complications in rectal surgery. Int J Colorectal Dis. 2007; 22:1499–1507. PMID: 17639424.

20. Kørner H, Nielsen HJ, Søreide JA, Nedrebø BS, Søreide K, Knapp JC. Diagnostic accuracy of C-reactive protein for intraabdominal infections after colorectal resections. J Gastrointest Surg. 2009; 13:1599–1606. PMID: 19479312.

21. Oberhofer D, Juras J, Pavicić AM, Rancić Zurić I, Rumenjak V. Comparison of C-reactive protein and procalcitonin as predictors of postoperative infectious complications after elective colorectal surgery. Croat Med J. 2012; 53:612–619. PMID: 23275327.

22. Mokart D, Merlin M, Sannini A, Brun JP, Delpero JR, Houvenaeghel G, et al. Procalcitonin, interleukin 6 and systemic inflammatory response syndrome (SIRS): early markers of postoperative sepsis after major surgery. Br J Anaesth. 2005; 94:767–773. PMID: 15849208.

23. Chakravarthy M, Kavaraganahalli D, Pargaonkar S, Hosur R, Harivelam C, Bharadwaj A, et al. Elevated postoperative serum procalcitonin is not indicative of bacterial infection in cardiac surgical patients. Ann Card Anaesth. 2015; 18:210–214. PMID: 25849691.

24. Lagoutte N, Facy O, Ravoire A, Chalumeau C, Jonval L, Rat P, et al. C-reactive protein and procalcitonin for the early detection of anastomotic leakage after elective colorectal surgery: pilot study in 100 patients. J Visc Surg. 2012; 149:e345–e349. PMID: 23102916.

25. Macrina F, Tritapepe L, Pompei F, Sciangula A, Evangelista E, Toscano F, et al. Procalcitonin is useful whereas C-reactive protein is not, to predict complications following coronary artery bypass surgery. Perfusion. 2005; 20:169–175. PMID: 16038389.
26. Silvestre J, Rebanda J, Lourenço C, Póvoa P. Diagnostic accuracy of C-reactive protein and procalcitonin in the early detection of infection after elective colorectal surgery - a pilot study. BMC Infect Dis. 2014; 14:444. PMID: 25132018.

27. Serejo LG, da Silva-Júnior FP, Bastos JP, de Bruin GS, Mota RM, de Bruin PF. Risk factors for pulmonary complications after emergency abdominal surgery. Respir Med. 2007; 101:808–813. PMID: 16963245.

28. McAlister FA, Bertsch K, Man J, Bradley J, Jacka M. Incidence of and risk factors for pulmonary complications after nonthoracic surgery. Am J Respir Crit Care Med. 2005; 171:514–517. PMID: 15563632.

29. Rao MK, Reilley TE, Schuller DE, Young DC. Analysis of risk factors for postoperative pulmonary complications in head and neck surgery. Laryngoscope. 1992; 102:45–47. PMID: 1731156.

Fig. 1
C-reactive protein (mg/L) changes in patients with and those without postoperative hospital-acquired pneumonia (HAP) (presented as the median). *Indicates significant difference between patients with and those without postoperative HAP. †Indicates significant difference compared to preoperative value of patients without postoperative HAP. ‡Indicates significant difference compared to preoperative value of patients with postoperative HAP. CRP: C-reactive protein, Pre-op: preoperative, POD: postoperative day, HAP: hospital acquired pneumonia.
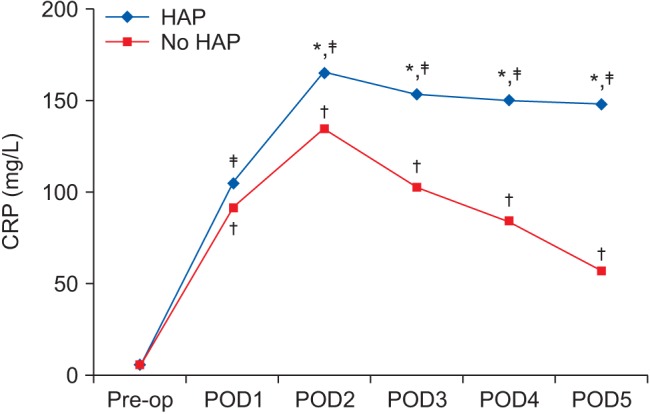
Fig. 2
Procalcitonin (ng/ml) changes in patients with and those without postoperative hospital-acquired pneumonia (presented as the median). *Indicates significant difference between patients with and those without postoperative HAP. †Indicates significant difference compared to preoperative value of patients without postoperative HAP. ‡Indicates significant difference compared to preoperative value of patients with postoperative HAP. PCT: procalcitonin, Pre-op: preoperative, POD: postoperative day, HAP: hospital acquired pneumonia.
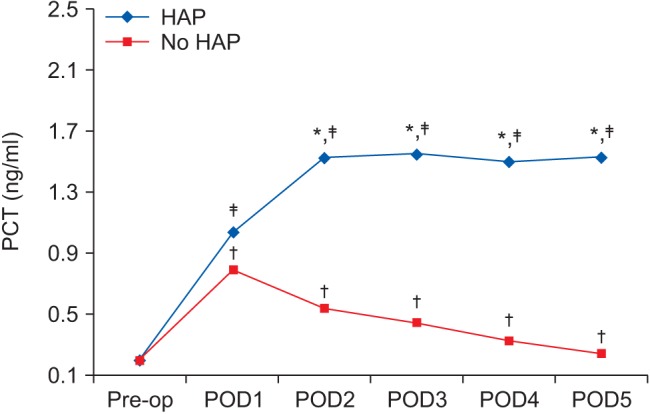
Fig. 3
The receiver operating characteristic curve for procalcitonin (ng/ml) in postoperative days 2–5. PCT: procalcitonin.
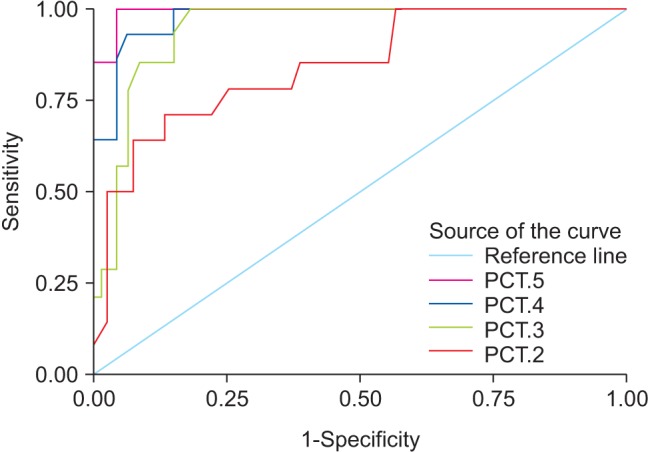
Fig. 4
The receiver operating characteristic curve for C-reactive protein (mg/L) in postoperative days 2–5. CRP: C-reactive protein.
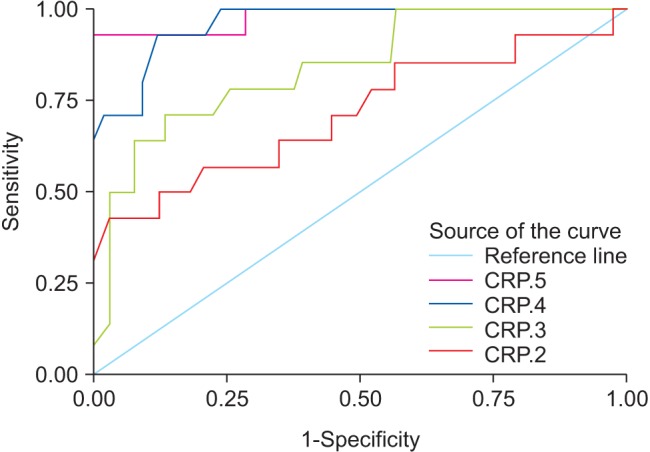
Table 1
Perioperative Patient Characteristics
Data are expressed as the mean ± SD or number of patients (%). The used tests were the chi-square test, Fisher's exact test, and t-test. HAP: hospital-acquired pneumonia, ASA: American Society of Anesthesiologists, BMI: body mass index, COPD: chronic obstructive pulmonary disease, ICU: intensive care unit.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download