This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Etomidate injection is often associated with myoclonus. Etomidate injection technique influences the incidence of myoclonus. This study was designed to clarify which of the two injection techniques—slow injection or priming with etomidate—is more effective in reducing myoclonus.
Methods
This prospective randomized controlled study was conducted on 189 surgical patients allocated to three study groups. Control group (Group C, n = 63) received 0.3 mg/kg etomidate (induction dose) over 20 s. Priming group (Group P, n = 63) received pretreatment with 0.03 mg/kg etomidate, followed after 1 min by an etomidate induction dose over 20 s. Slow injection group (Group S, n = 63) received etomidate (2 mg/ml) induction dose over 2 min. The patients were observed for occurrence and severity of myoclonus for 3 min from the start of injection of the induction dose.
Results
The incidence of myoclonus in Group P (38/63 [60.3%], 95% CI: 48.0–71.5) was significantly lower than in Group C (53/63 [84.1%], 95% CI: 72.9–91.3, P = 0.003) and Group S (49/63 [77.8%], 95% CI: 66.0–86.4, P = 0.034). Myoclonus of moderate or severe grade occurred in significantly more patients in Group C (68.3%) than in Group P (36.5%, P < 0.001) and Group S (50.8%, P = 0.046), but the difference between Groups P and S was not significant (P = 0.106).
Conclusions
Priming is more effective than slow injection in reducing the incidence of myoclonus, but their effects on the severity of myoclonus are comparable.
Keywords: Adverse effect, Etomidate, Injection method, Myoclonus
Introduction
Etomidate is an imidazole derivative, that is widely used as an intravenous anesthetic induction agent. It causes minimal respiratory depression and does not induce histamine release. Being a cardio-stable drug, it is especially preferred in patients who are hemodynamically unstable. However, the use of etomidate may be associated with undesirable effects such as myoclonus. The incidence of etomidate-induced myoclonus (EIM) in unpremedicated patients is reported to be as high as 50–80% [
1–
3]. The myoclonic movements are not only disturbing [
4] but may increase the risk of regurgitation and aspiration in non-fasted patients [
5], and are detrimental in patients with open-globe injury [
6]. Altering the speed of injection of etomidate [
4] and pre-treatment with drugs such as lidocaine [
7], midazolam [
8], magnesium [
9], fentanyl [
10], and dexmedetomidine [
11] have been investigated as ways of reducing EIM, with variable results.
A change in the etomidate injection technique can itself reduce the incidence of EIM, eliminating the need for an additional drug with its inherent cost and potential side effects [
2,
4]. Pre-treatment with a low dose of etomidate (priming dose) [
2] and slow injection of the induction dose [
4] have both been shown to reduce the incidence of EIM. To the best of our knowledge, however, there are no studies comparing these two techniques, and therefore it is not clear which is the better option. We, therefore, conducted this study to determine which of the techniques is more effective in reducing the incidence of myoclonus.
Materials and Methods
This prospective, randomized controlled study was conducted in the Department of Anesthesia and Intensive Care, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India, after obtaining approval from the hospital ethics committee. The study was registered with the Clinical Trials Registry—India (CTRI/2015/02/005592).
The study was conducted from November 2013 to May 2015, on 189 patients ranging in age from 18 to 60 years, of both sexes, American Society of Anesthesiologists physical status classification I–II, scheduled for elective surgery under general anesthesia. The surgical procedures included general surgery, urosurgery, gynecological surgery, plastic surgery, and ear-nose-throat (ENT) surgery. Exclusion criteria were: pre-existing adrenal disease or adrenocortical insufficiency; receiving or having a history of receiving steroids within the last three months; sepsis; hypersensitivity to the study drug; history of a seizure disorder; and neurological disease.
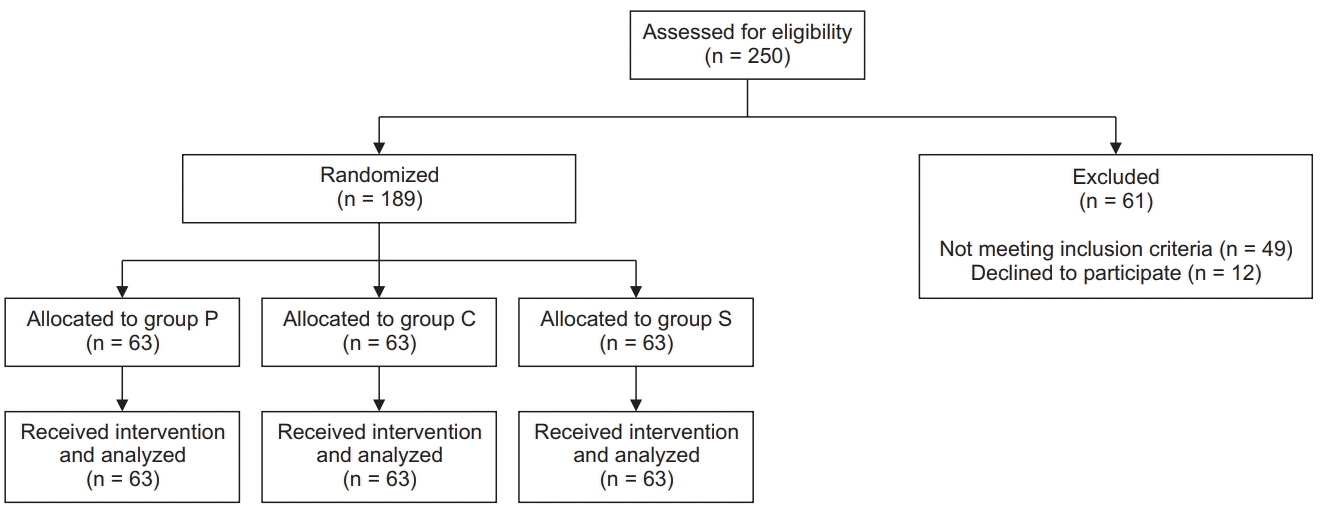
A total of 250 patients were assessed for eligibility. Of these, 61 patients were excluded, as 49 patients did not meet the inclusion criteria and 12 patients declined to participate. Finally, 189 patients were randomized into the three study groups, with 63 patients in each group (
Fig. 1).
Patients were advised to fast for 6 h prior to surgery and were not given any premedication. Written informed consent was obtained from all patients after explanation of the risks and benefits of the study medication and the anesthesia technique.
Using a computer-generated random number table, patients were allocated randomly into three groups (63 patients in each group), the control group (Group C), priming group (Group P), and slow injection group (Group S). Concealment of allocation was done using sequentially numbered, opaque, sealed envelopes, which were numbered in advance and opened only after the participant’s name and other details were written on the appropriate envelope.
In the operating room, standard monitors were applied (electrocardiogram, non-invasive blood pressure, and pulse oximeter) and baseline hemodynamic parameters were noted. Intravenous access was secured with an 18-gauge intravenous catheter. In Group P, patients received a priming dose of 0.03 mg/kg etomidate, followed after 1 min by an induction dose of 0.3 mg/kg, injected manually over 20 s. In Group S, patients received an induction dose of 0.3 mg/kg etomidate, injected slowly over 2 min using a syringe infusion pump. In Group C, patients received an induction dose of 0.3 mg/kg etomidate, injected manually over 20 s.
All patients were watched for the occurrence of myoclonus (primary endpoint) by an independent observer for 3 min from the start of injection of the induction dose of etomidate. The time of onset of myoclonus was noted and categorized as ‘within 1 min,’ ‘1 to 2 min,’ and ‘2 to 3 min’ from the start of induction. The myoclonic movement was graded as 0 for no myoclonus, 1 for mild myoclonus (short movement of a body segment, e.g., a finger or wrist), 2 for moderate myoclonus (mild movement of two different muscle groups, e.g., face and arm), and 3 for severe myoclonus (intense myoclonic movement in two or more muscle groups or fast adduction of a limb) [
4]. In case of difficulty in mask ventilation due to myoclonus, we planned to administer a neuromuscular blocking agent immediately. The time until loss of consciousness (LOC), defined as the time from start of injection of the induction dose of etomidate until loss of response to verbal commands (e.g., noncompliance when asked to open the eyes), was noted. The dose of etomidate administered when LOC was observed (LOC dose) was also noted.
Three min after the start of induction with etomidate, patients in all the groups were administered fentanyl (2 μg/kg) and vecuronium bromide (0.1 mg/kg) to facilitate tracheal intubation. Anesthesia was maintained with isoflurane in a mixture with oxygen and nitrous oxide. After completion of surgery, residual neuro-muscular blockade was antagonized with neostigmine and glycopyrrolate.
Heart rate, systolic, diastolic, and mean arterial pressure, and peripheral oxygen saturation were recorded every minute from the start of induction until tracheal intubation and at 1, 2, and 5 min after tracheal intubation (secondary endpoints).
Statistical analysis
Previous studies have estimated the incidence of EIM to be around 50% [
1–
3]. The sample size calculated for a 5% level of significance and power of 80% was 57 in each group, assuming that the proportion of patients who developed myoclonus after slow injection or pre-treatment with etomidate would be 25%. Assuming a 10% dropout rate, the total sample size was set at 189 (a minimum of 63 in each group).
Statistical analysis was performed using SPSS for Windows, version 17.0 (SPSS Inc., USA). Continuous variables—age, weight, induction dose, LOC dose, LOC time, duration of surgery, heart rate and blood pressure—are presented as mean (SD), and categorical variables—American Society of Anesthesiologists (ASA) class, sex distribution, myoclonus incidence, severity grade, and time of onset noted as occurrence of myoclonus within a categorical time frame (1 min, 1 to 2 min, and 2 to 3 min)—are presented as absolute numbers or percentages with 95% CI. The continuous data were first checked for normality using the Shapiro-Wilk test. All the continuous variables were found to be normally distributed. Therefore, all these variables were compared across the three groups using ANOVA. The F-values were found statistically insignificant for all continuous variables except LOC dose and LOC time. Tukey’s multiple comparisons test was used for intergroup comparisons for LOC dose, as the variances were homogenous. Tamhane’s T2 test was used for intergroup comparisons for LOC time, as the variances were not homogenous. Categorical variables were analyzed using the chi-square test. For all statistical tests, P < 0.05 was taken to indicate a significant difference.
Results
There were no significant differences in demographic profile, the mean duration of surgery, or dose of etomidate required for induction among the groups (
Table 1).
The mean LOC dose was significantly lower in Group S than in Group P and Group C. There was no difference in mean LOC dose between Group P and Group C. The mean time until LOC in Group S was significantly longer than that in Group C and Group P. There was no difference in mean time until LOC between Group P and Group C (
Table 1).
The incidence, severity, and time of onset of myoclonus in the three groups were as shown in
Table 2. The incidence of myoclonus was significantly lower in Group P (60.3%, 95% CI: 48.0–71.5) than in Group C (84.1%, 95% CI: 72.9–91.3, P = 0.003) and Group S (77.8%, 95% CI: 66.0–86.4, P = 0.034). There was no significant difference in the incidence of myoclonus between Groups C and S (P = 0.364).
A moderate or severe grade of EIM (Grade 2 or 3) was observed in a significantly greater number of patients in Group C than in Group P and Group S. However, no significant difference in the number of patients experiencing moderate to severe EIM was observed between Group P and Group S.
The time of onset of myoclonus, defined by the occurrence of myoclonus within the previously specified time frames was as shown in
Table 2. Myoclonus occurred within 2 min from the start of induction in 44/53 patients (83.0%), 36/49 patients (73.5%), and 27/38 patients (71.1%) in Groups C, S, and P respectively, with no significant differences between the groups. Myoclonic movement was observed beyond the 3-min time frame of observation in four patients in Group P and six patients in Group S (
Table 2).
Hemodynamic parameters such as mean arterial lpressure (
Fig. 2) and heart rate (
Fig. 3) were comparable across the three groups. None of the patients showed any incidence of oxygen desaturation.
Discussion
In the present study, we found that a small priming dose (0.03 mg/kg) of etomidate given prior to the induction dose was more effective than slow injection of etomidate (over 2 min) in reducing the incidence of EIM.
The involuntary myoclonic movements seen with etomidate are believed to be caused by subcortical disinhibition [
1]. A large dose of etomidate depresses the cortical activity before depressing subcortical activity, thereby causing myoclonus [
8,
12,
13]. Both pretreatment (priming) with etomidate and slow administration may prevent the unsynchronized onset of drug action at different sites within the central nervous system that may be responsible for EIM [
1,
4].
In our study, the incidence of EIM in the priming group was significantly lower than in the control and slow injection groups. There was no significant difference in the incidence of EIM between the control and slow injection groups. The incidence of 84.1% (53/63 patients) EIM in the control group in our study is comparable to the results of other studies [
2,
10]. Aissaoui et al. [
2] observed a significant reduction in the incidence of EIM following pretreatment with a low dose of etomidate (priming dose). In their study, 26% (6/23 patients) of patients in the priming group experienced myoclonus as compared to 87% (20/23 patients) in the control group (P < 0.001). Doenicke et al. [
1], in a crossover study involving eight patients, found that the incidence of EIM was 25% (2/8 patients) when etomidate induction followed pretreatment with 0.05 mg/kg etomidate, as compared to 75% (6/8 patients) in the control group. In another study, three different pretreatment doses of etomidate were compared. The incidence of EIM was found to be 20% (4/20 patients), 25% (5/20 patients), and 35% (7/20 patients) for pretreatment with 0.03, 0.05, and 0.075 mg/kg etomidate, respectively [
1]. We found a higher incidence of EIM in the priming group than reported by the other studies [
1,
2]. This could perhaps be a result of the fact that we did not premedicate our patients and administered a muscle relaxant at 180 s after the start of induction, thus allowing a longer time period for observation of myoclonus. In contrast, Doenicke et al. [
1] premedicated their patients with oral midazolam 1 h prior to induction and administered a muscle relaxant at 90 s after induction, allowing a shorter time period for observation than ours. Aissaoui et al. [
2] also used a smaller time period for observation, as they administered a muscle relaxant at 60 s after induction with etomidate.
Do et al. [
4] observed that slow injection of etomidate (over 2 min) resulted in a significantly lower incidence of EIM (28% [7/25 patients]) than giving it as a fast injection over 10 s (84% [21/25 patients], P < 0.001). Even though our study methodology was similar to that of Do et al., we observed a higher incidence (77.8% [49/63 patients]) of EIM in the slow injection group. This difference could perhaps be attributed to the fact that our study population was younger (mean [SD] age, 34.6 [10.8] vs. 52 [14] years). The age of a patient affects the risk of myoclonus: the younger the patient, the higher the risk [
5].
We found that the percentage of patients who experienced moderate or severe EIM was significantly lower in the priming and slow injection groups than in the control group. Aissaoui et al. [
2] also found the severity of myoclonus to be significantly lower with priming than in a control group. Do et al. [
4] found the severity of myoclonus to be significantly lower in a slow injection group than in a fast injection group. We found no significant difference in the severity of myoclonus between the priming and slow injection groups. To the best of our knowledge, no study has compared the effects of priming vs. slow injection of etomidate on the incidence of EIM. We are hence unable to compare our results with those of other authors.
Do et al. [
4] noted that in their slow injection group, in contrast with the fast injection group, LOC occurred before the complete induction dose was administered. This finding implies the possibility of a smaller dose requirement in the slow injection group [
4]. In our study, the mean time until LOC was significantly longer in Group S than in Group C and Group P. By the time LOC occurred in Group S, only 10.8 (3.4) mg of etomidate had been administered, while the complete induction dose was 16.7 (3.4) mg. On the other hand, in the priming and control groups, the complete induction dose was administered before LOC was observed. The occurrence of EIM is dose-related, larger doses being associated with a higher incidence [
1]. Had etomidate been administered until the onset of LOC, instead of as at a fixed dosage, it is possible that the incidence of EIM would have been reduced in the slow injection group.
In the majority of the patients in all our study groups, the onset of myoclonus occurred within 2 min after the start of induction. However, four patients in the priming group and six patients in the slow injection group had myoclonus after 3 min from the start of induction. The period of observation for myoclonus varied from 1 to 3 min in most previous studies, suggesting that the actual incidence of EIM may be higher than that reported. Delayed myoclonic movements could go undetected due to masking by a neuromuscular blockade. To determine the true incidence of EIM, further studies are needed to identify the optimal observation period.
Our study has several limitations. First, it was not a blind study. Due to the variable speed of injection (2 min vs. 20 s) of etomidate in the study groups, it was not possible to blind either the physicians or the patients. Second, the time of onset of myoclonus was categorized into 1-min intervals from the start of induction. Thus, the exact time of onset and duration of myoclonus were not noted.
To conclude, we found that priming with etomidate significantly reduces the incidence and severity of myoclonus. Priming is more effective than slow injection in reducing the incidence of myoclonus, but their effects on the severity of myoclonus are comparable.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download