Abstract
Background
Thalidomide has been recognized as having an anti-allodynic effect against neuropathic pain induced by spinal nerve ligation. Its clinical beneficial effects are mainly derived from its immune-modulating property, which is known to influence the analgesic action of morphine. The possible characteristics of systemic interactions between thalidomide and morphine in the context of spinal nerve ligation-induced neuropathic pain were examined in rats.
Methods
Neuropathic pain was induced by ligation of the L5/6 spinal nerves in male Sprague-Dawley rats and mechanical allodynia was assessed using von Frey filaments. The ED50 was calculated for thalidomide and for morphine, and the mixture of both drugs was intraperitoneally administered at different doses of ED50 of each drug (1/8, 1/4, 1/2, 1/1 of ED50) to obtain the experimental ED50 value for the combination of thalidomide and morphine. Isobolographic analysis was used to evaluate the characteristics of drug interactions between morphine and thalidomide.
Results
The ED50 of thalidomide was three-fold higher than that of morphine. The experimental ED50 value of the mixture of thalidomide and morphine was significantly lower than the calculated theoretical ED50 value. Isobolographic analysis revealed a synergistic interaction for anti-allodynic effect after intraperitoneal delivery of the thalidomide-morphine mixture.
Conclusions
These results suggest that thalidomide acts synergistically with morphine to produce an anti-allodynic effect in neuropathic pain induced by spinal nerve ligation in rats. Thus, the combination of thalidomide with morphine may be one of the useful strategies in the management of neuropathic pain.
Neuropathic pain was recently redefined as "pain arising as a direct consequence of a lesion or disease affecting the somatosensory system" [1]. It is associated with severe, chronic sensory disturbances characterized by spontaneous pain (ongoing, paroxysms) and evoked types of pain (hyperalgesia, allodynia). The current treatment options are insufficient to manage the pain effectively and make it endurable. It was reported that neuropathic pain could be reduced by only 30-50% with the available treatment in no more than 50% of patients [2], which could be attributable to the lack of knowledge of the mechanisms of the neuropathic pain.
Much evidence suggests that activation of glial cells such as astrocytes or microglia in the spinal cord resulting from peripheral nerve injury is involved in the pathogenesis of neuropathic pain [3,4]. It has been demonstrated that both microglia and astrocytes could release a variety of pro-inflammatory cytokines, which are one of the major components mediating or maintaining hyperalgesia and allodynia [5,6]. Opioids are among the most powerful analgesics used to treat nociceptive pain. It is not fully understood, however, why opioids are less effective in the treatment of neuropathic pain compared to acute or inflammatory pain. In many studies, glial activation has been proven to be related to analgesic properties of opioids such as tolerance and hyperalgesia [7,8].
The findings described above suggest that there are many similarities between the mechanisms of developing and maintaining neuropathic pain and lowering the efficacy of morphine in neuropathic pain. Moreover, glial cells are reported to play an important role in the generation of neuropathic pain and the modulation of the other glial cells, and thus neuroimmune activation; glia may provide a strong therapeutic mechanism for increasing morphine efficiency and preventing morphine tolerance during neuropathic pain [9-11]. Thalidomide, a derivative of glutamic acid, has several immune-modulating properties, such as suppressing the synthesis and release of pro-inflammatory cytokines, and increasing the release such as anti-inflammatory cytokines [12,13]. Its efficacy in the treatment of neuropathic pain was also shown in previous reports [13-15].
The purpose of the present study was to evaluate the characteristics of pharmacological interactions between systemically administered thalidomide and morphine in neuropathic pain induced by spinal nerve ligation in rats.
All procedures were approved by the Institutional Animal Care and Use Committee at our university (CNU IACUCH-2010-2). Male Sprague-Dawley rats weighing 100-120 g were used. They were housed under a 12 hour day/night cycle with unrestricted access to food and water. From the day of surgery, all rats were housed in individual cages with sawdust bedding.
The L5,6 spinal nerve ligation model was used as previously described [16]. Surgical procedures were performed under general anesthesia with the volatile anesthetic enflurane. After sterilization of the lower back with antiseptics, a midline incision was made, with the center of the incision at the level of the iliac crest. Through careful dissection of the paraspinal muscles, the left L5 and L6 nerves were isolated and tightly ligated distal to the dorsal root ganglion with 6-0 silk suture. The muscles and skin were closed layer by layer, and the rats were allowed to recover for 10 days prior to behavioral testing. Rats showing motor impairment after surgery were euthanized immediately by overdose of anesthetics.
Normal motor function was confirmed by measuring the righting reflex and the placing/stepping reflex. The righting reflex was examined by placing the rat horizontally with its back on the table. The animals normally return to the upright position by an immediate coordinated twisting of the body. The placing/stepping reflex was evaluated by drawing the dorsum of either hindpaw across the edge of the table. Normally, rats continue to place the paw forward into a walking position. The pinna reflex and corneal reflex were also checked to verify normal sensory function, and judged to be either present or absent. All of the above tests were performed after the surgery and after intraperitoneal injection of the experimental drugs.
Allodynic response to mechanical stimulation with von Frey filaments was assessed as described previously to determine the 50% probability paw withdrawal threshold (PWT) [17]. Rats were placed individually in transparent Plexiglass chambers with wire mesh floors and allowed to acclimate for 30 min. The PWT in response to mechanical stimulation was measured using the up-and-down method. A series of eight von Frey filaments (0.4, 0.7, 1.2, 2.0, 3.6, 5.5, 8.5, and 15 g) were vertically applied to the plantar surface of the hind paw for 5 sec so that the hair was bent. Rapid withdrawal, licking or flinching of the paw was considered a positive response. For rats showing positive responses, a stiffer filament was applied in the next trial; for those showing no withdrawal or licking, a less stiff filament was used. To avoid damage to the paw, a withdrawal threshold of 15 g was set for the cutoff value. If animals did not withdraw or lick their paw on the application of a von Frey filament of 15 g, they were assigned a PWT of 15 g. However, animals with a PWT higher than 5 g were regarded as having a failure of neuropathic pain development, and were not included in this study.
Thalidomide (Sigma Chemical Co., St. Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO) and the resulting solution diluted with saline to reduce the concentration of DMSO to 10%. Morphine was dissolved in normal saline. Both agents were administered intraperitoneally.
After a period of acclimation and measurement of baseline PWT, the animals were injected intraperitoneally with the experimental drugs in a volume of 2 ml. Measurement of mechanical allodynia with von Frey filament was performed 30 min after the drug injection. The behavioral testing was performed only by the first author of this manuscript, who was not informed of the drugs previously injected to the test animals. The withdrawal threshold data from von Frey filament testing was converted to % maximal possible effect (MPE), which was obtained from the following formula: %MPE = [(post-drug threshold - post-injury baseline threshold)/(cutoff threshold - post-injury baseline threshold)] × 100. %MPE was used to calculate the ED50 of each drug.
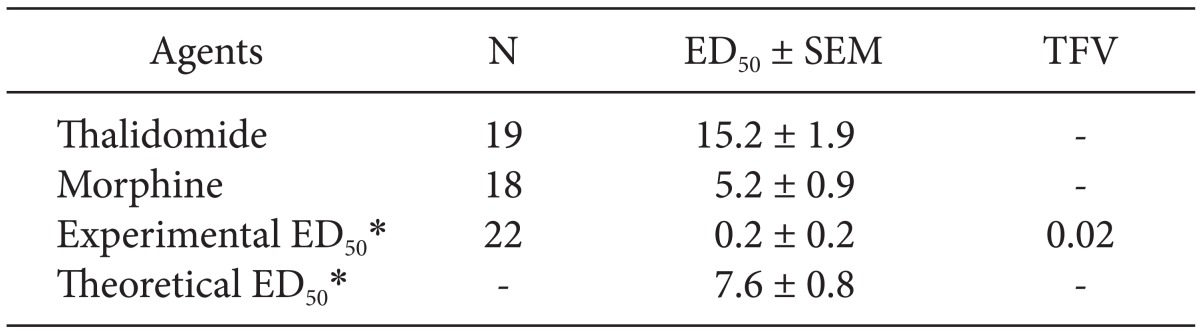
ED50 values for thalidomide were obtained from the results of a study of our laboratory which reported that intraperitoneally administered thalidomide significantly reduced the paw withdrawal threshold and produced dose-responsiveness [15]. To obtain the ED50 of morphine, the additional doses of morphine (30 mg and 100 mg/kg) were intraperitoneally administered and the PWT in response to mechanical stimulation was measured. Both doses of morphine produced an anti-allodynic effect, but the data from animals treated with 100 mg/kg was not included in determination of the ED50 of morphine because they were found to be sedated after morphine injection. The results of our previous study [15] and the above additional experiment showed a dose-dependent anti-allodynic effect of intraperitoneal morphine (3, 10 and 30 mg/kg), in which the MPE for each of the doses was 22.2% (P < 0.05 vs. control), 85.3% (P < 0.01 vs. control) and 100.0% (P < 0.001 vs control), respectively. Taken together, ED50 (95% confidence interval) values of thalidomide (6.25, 12.5, 25 and 50 mg/kg) and morphine (3, 10 and 30 mg/kg) were 15.2 (11.7-19.8) mg/kg or 5.2 (3.7-7.4) mg/kg, respectively.
To evaluate the characteristics of interaction between thalidomide and morphine, a mixture of both drugs was intraperitoneally administered in rats that showed mechanical allodynia after spinal nerve ligation. Four different doses of 1/8, 1/4, 1/2, or 1/1 of the ED50 of morphine were prepared and then each preparation of morphine was added into thalidomide which was prepared at a dose of 1/8, 1/4, 1/2 or 1/1 of the ED50, respectively. The interaction between thalidomide and morphine in antinociception was evaluated by isobolographic analysis, which is based on the comparison of doses determined as being equally effective [18].
After obtaining the ED50 of the mixture (experimental ED50), an isobologram was constructed by plotting the ED50 values of the individual agents on the x- and y-axes. Then, the theoretical additive combined dose was calculated and individual variances for the combinations of agents were obtained. In addition, the total fraction value was calculated as follows to describe the magnitude of the interaction: Total fraction value = (ED50 for the combination of thalidomide and morphine/ED50 for thalidomide alone) + (ED50 for the combination of thalidomide and morphine/ED50 for morphine alone). The fractional value indicates the portion of the single ED50 value that the corresponding ED50 value for the combination accounted for. For an additive interaction, the value will be close to 1. Antagonistic interactions produced values > 1; synergistic interactions produced values < 1.
Data are expressed as the mean ± SEM. To analyze the difference in PWT according to dose change of the mixiture, one-way analysis of variance (ANOVA) and Scheffe's multiple comparison test were used. Differences between theoretical and experimentally determined ED50 values were compared using t-tests. A value of P < 0.05 was considered to be significant.
After ligation, the withdrawal threshold in the ipsilateral paw of spinal nerve-ligated animals was significantly reduced compared to that of the contralateral paw. The decreased withdrawal threshold of the ipsilateral paw did not change during the observation period (Fig. 1). The pharmacological treatments of thalidomide and morphine produced no motor impairment in experimental rats, as assessed by the righting and placing/stepping reflexes.
The combination of each drug at a dose of 1/8, 1/4, 1/2, and 1/1 ED50 of morphine and thalidomide produced a significant anti-allodynic effect compared to baseline. The PWT of animals injected with the combination of morphine and thalidomide at a dose of ED50 was significantly different from that of animals injected with a dose of 1/8 of ED50 (Fig. 2).
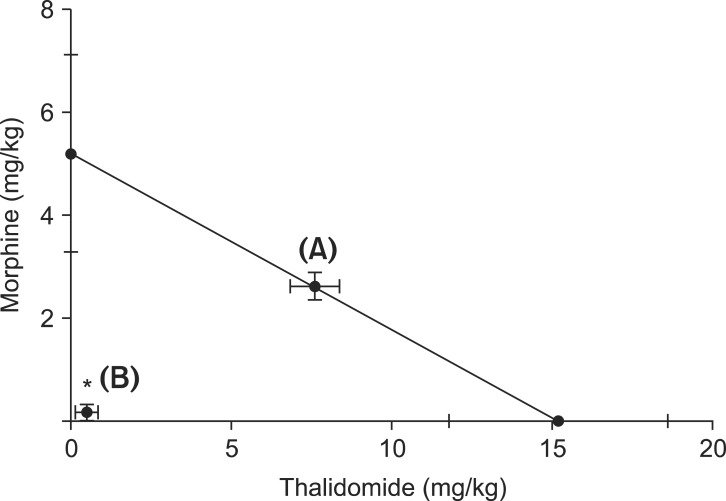
The ED50 values for thalidomide was 3-fold larger than that of morphine (Table 1). Isobolographic analysis revealed a synergistic interaction between intraperitoneally administered thalidomide and morphine in neuropathic pain induced by spinal nerve ligation. The experimental ED50 values were significantly lower than the calculated ED50 values (Fig. 3). The total fraction value of less than 1 indicated a synergistic interaction (Table 1).
The current study demonstrated a synergistic interaction in the anti-alloidynic effect between thalidomide and morphine by isobolographic analysis, which suggests that the antiallodynic effect of morphine could be augmented by adding thalidomide to a drug regimen in a neuropathic pain state.
Originally, allodynia and hyperalgesia were thought to solely originate from the dysfunction of neurons. Many studies over 20 years have shown that the mechanism underlying the development and maintenance of neuropathic pain is complex and involves the interaction of neuronal and nonneuronal cells [3-5]. Not only neurotransmitters from synaptic neurons, but pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 released from glial cells are involved in the development of hyperalgesia and allodynia in inflammatory and neuropathic pain [6,19]. These molecules have been proposed as drug targets for the treatment of neuropathic pain [20]. TNF-α is regarded as an important initiating factor and a crucial maintenance factor in the pathology of neuropathic pain [6,21]. A previous study by Beattie et al. [22] described that TNF-α could influence the functional status of neuronal cells in an indirect way, in which endogenous TNF-α enhances the expression of glutamate AMPA receptors on the membrane of neurons, thereby increasing the synaptic transmission.
It has been reported that opioid potency is reduced by many mechanisms in neuropathic pain. Some of these mechanisms are neuronal, but others are related to cells of the nervous system other than neurons, which are mainly contributed by glial cells and pro-inflammatory cytokines including TNF-α [23,24]. Repeated administration of morphine induced the activation of glia, and the activated glial cells of the spinal cord could produce the neuroexcitatory pro-inflammatory cytokines including TNF-α, IL-1, and IL-6 [23]. Additionally, since it is known that the opioid-induced glial activation opposes opioid analgesia, some glial inhibitors are proposed as potential useful co-analgesic agents for opioid treatment of neuropathic pain [11]. The results of many studies support the idea that modulation of glial and neuroimmune activation may be a potential therapeutic mechanism for enhancement of morphine analgesia.
Glial activation is consistently observed in various models of neuropathic pain. Accumulating data suggests that peripheral nerve injury leads to activation of astrocytes, which results in systemic release of pro-inflammatory mediators (e.g., TNF-α and IL-1β) in the spinal cord and the brain, which in turn is associated with the persistence of hypersensitivity in chronic neuropathic pain [3-6]. Mika et al. [25] reported that microglial cells are strongly activated in the animal neuropathic pain model both in the dorsal root ganglia and at the spinal level after peripheral nerve injury. Also, it is already known that activated microglial cells produce pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, which mediate allodynia and hyperalgesia [5,6].
Although thalidomide has broad-spectrum immunologic effects, their major mode of action is the inhibition of TNF-α synthesis through enhanced TNF-α mRNA degradation [26]. Recent studies reported that thalidomide inhibits inflammatory hyperalgesia in rats and reversed the hyperalgesia in chronic sciatic nerve injury by inhibiting TNF-α production [13,14]. The above observations suggest that the decreased TNF-α levels produced by thalidomide may lead to the enhancement of morphine efficacy.
Various factors are involved in drug interactions, and elucidating the underlying mechanisms of the synergistic interaction between morphine and thalidomide in this behavioral study was difficult. First, drugs may interact by altering each other's kinetics. One agent may alter the actions of the other agent at receptors or channels. Second, the synergistic interaction may stem from dual -block at two different points. Morphine and thalidomide act at different sites, with morphine acting mainly on neurons and thalidomide exerting its effect on glial cells. The synergistic interaction might be postulated to be derived from the dual block at two different points by thalidomide and morphine.
Another clue to the mechanism of the synergism in this study could be seen in the study by Schafers et al. [27], in which exogenous application of TNF-α to DRG neurons synergized with nerve injury producing allodynia of faster onset and enhanced intensity, even at the subthreshold dose which did not elicit allodynia when applied in naive rats. In addition, pretreatment with etanercept, a TNF inhibitor attenuated this effect of exogenous TNF-α. These findings suggest that blocking the effects of TNF-α could augment the efficacy of analgesics which act at nociceptive sensory transmission of the spinal cord. However, it is not possible to determine the synergistic interaction observed in the current study using pharmacological and behavioral testing. The understanding of the precise mechanisms of the synergistic interaction between thalidomide and morphine needs further fine investigations with biochemical, molecular and electrophysiological tests.
Drugs with the effect of modulating cytokines are currently available, but their use has been limited by concomitant systemic side effects. The synergism produced by morphine and thalidomide could serve as an alternative method of applying cytokine-modulating drugs to the treatment of neuropathic pain. However, similar studies on various animals are required to obtain a proper understanding of the effects of thalidomide on peripheral and central nerve injury-induced neuropathic pain.
In conclusion, thalidomide acts synergistically with morphine in neuropathic pain induced by spinal nerve ligation in rats. Thus, the combination of thalidomide with morphine may be used as one of the combination strategies in the management of neuropathic pain states.
Acknowledgments
This study was supported by a grant (CRI 12 038-1) of Chonnam National University Hospital Biomedical Research Institute.
References
1. Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical research purposes. Neurology. 2008; 70:1630–1635. PMID: 18003941.
2. Wallace JM. Update on pharmacotherapy guidelines for treatment of neuropathic pain. Curr Pain Headache Rep. 2007; 11:208–214. PMID: 17504648.

3. Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci STKE. 2004; 2004:reE14. PMID: 15454629.

4. Watkins LR, Milligan ED, Maier SF. Spinal cord glia: new players in pain. Pain. 2001; 93:201–205. PMID: 11514078.

5. Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001; 24:450–455. PMID: 11476884.

6. White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci U S A. 2007; 104:20151–20158. PMID: 18083844.

7. Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A. 1999; 96:7731–7736. PMID: 10393889.

8. Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002; 22:9980–9989. PMID: 12427855.

9. Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001; 24:450–455. PMID: 11476884.

10. White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci U S A. 2007; 104:20151–20158. PMID: 18083844.

11. Mika J. Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol Rep. 2008; 60:297–307. PMID: 18622054.
12. Ribeiro RA, Vale ML, Ferreira SH, Cunha FQ. Analgesic effect of thalidomide on inflammatory pain. Eur J Pharmacol. 2000; 391:97–103. PMID: 10720640.

13. George A, Marziniak M, Schäfers M, Toyka KV, Sommer C. Thalidomide treatment in chronic constrictive neuropathy decreases endoneurial tumor necrosis factor-alpha, increases interleukin-10 and has long-term effects on spinal cord dorsal horn met-enkephalin. Pain. 2000; 88:267–275. PMID: 11068114.
14. Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain. 2006; 123:306–321. PMID: 16675114.

15. Choi JI, Kim WM, Yoon MH, Lee HG. Antiallodynic effect of thalidomide and morphine on rat spinal nerve ligation-induced neuropathic pain. Korean J Pain. 2010; 23:172–178. PMID: 20830262.

16. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992; 50:355–363. PMID: 1333581.
17. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63. PMID: 7990513.

18. Yoon MH, Yaksh TL. Evaluation of interaction between gabapentin and ibuprofen on the formalin test in rats. Anesthesiology. 1999; 91:1006–1013. PMID: 10519504.

19. Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005; 6:521–532. PMID: 15995723.

20. Myers RR, Campana WM, Shubayev VI. The role of neuroinflammation in neuropathic pain: mechanisms and therapeutic targets. Drug Discov Today. 2006; 11:8–20. PMID: 16478686.

21. Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005; 6:521–532. PMID: 15995723.
22. Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, et al. Control of synaptic strength by glial TNF alpha. Science. 2002; 295:2282–2285. PMID: 11910117.
23. Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005; 28:661–669. PMID: 16246435.

24. Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001; 81:299–343. PMID: 11152760.

25. Mika J, Rojewska E, Makuch W, Przewlocka B. Minocycline reduces the injury-induced expression of prodynorphin and pronociceptin in dorsal root ganglion in rat model of neuropathic pain. Neuroscience. 2010; 165:1420–1428. PMID: 19961904.
26. Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor a by enhancing mRNA degradation. J Exp Med. 1993; 177:1675–1680. PMID: 8496685.
27. Schäfers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. 2003; 23:3028–3038. PMID: 12684490.
Fig. 1
Change in paw withdrawal threshold in spinal nerve-ligated rats. After the surgery, PWT of ipsilateral side of spinal nerve ligation is significantly decreased compared to contralateral side. *P < 0.01 vs. contralateral.
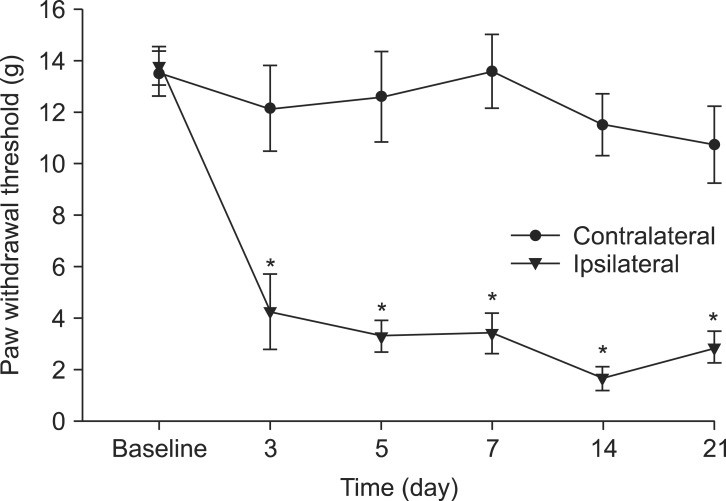
Fig. 2
Paw withdrawal threshold was significantly increased by the administration of mixture of thalidomide and morphine compared to baseline. The maximal possible effect (MPE, %) was increased as the proportion of combination dose to ED50 values for each drug was increased. *P < 0.01 vs. baseline, †P < 0.01 vs. 1/8 (proportion of mixture to ED50).
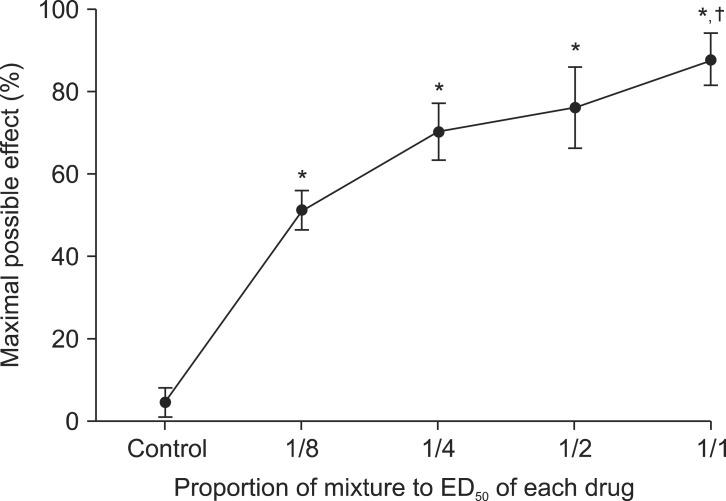
Fig. 3
Isobologram for the interaction between thalidomide and morphine is depicted. The ED50 values for each drug are plotted on the x- and y-axes. Dark circle, mean; bar, SEM. The straight lines connecting ED50 values indicate the combinations of drugs that have the same effect as the ED50 value of individual drugs in the case of an additive interaction. The points on this line indicate the theoretical additive ED50 values (A). The horizontal and vertical bars represent SEM. The experimental ED50 value (B) was significantly different from the theoretical ED50, indicating a synergistic interaction. *P < 0.01 vs. theoretical (A).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download