Abstract
Purpose
Maintenance therapy after oxaliplatin withdrawal is useful in patients with metastatic colorectal cancer (mCRC). This study aimed to investigate the timing of discontinuation or reintroduction of oxaliplatin and the optimal maintenance therapy regimen for survival.
Materials and Methods
PubMed and conference abstracts were searched to select phase II and III trials of first-line oxaliplatin-containing therapy with or without bevacizumab using maintenance therapy for mCRC. Correlations of median overall survival (OS) with induction therapy regimens, induction therapy duration, maintenance therapy regimens (fluoropyrimidine plus bevacizumab [FP+Bev], FP/Bev alone, and no treatment), and oxaliplatin reintroduction were investigated using correlation and weighted multivariate regression analyses.
Results
Twenty-two treatment arms were analyzed, including 2,581 patients. The maintenance therapy regimen FP+Bev showed the strongest correlation with a prolonged OS (Spearman’s partial correlation coefficient=0.42), and the other three variables correlated weakly with the OS. The maintenance therapy regimen significantly interacted with the induction chemotherapy duration (p=0.019). The predicted OS for FP+Bev crossed the lines of FP/Bev alone at 18 weeks of induction therapy, and of no treatment at 23 weeks. The corresponding OS at 12 and 27 weeks of induction therapies were 28.6 and 24.2 months for FP+Bev, 25.9 and 28.8 months for FP/Bev alone, and 20.5 and 27.5 months for no treatment.
Oxaliplatin combined with fluoropyrimidine (FP) is recognized as one of the standard first-line chemotherapies in patients with metastatic colorectal cancer (mCRC) [1–4]. The current recommendation is to add targeted agents such as bevacizumab or anti–epidermal growth factor receptor (EGFR) antibody to the oxaliplatin-containing therapy regimen [5–7]. Prolonged use of oxaliplatin is problematic because it induces chronic and cumulative peripheral neuropathy. Despite clinical trials examining prophylactic or therapeutic agents for treating oxaliplatin-induced acute/chronic peripheral neuropathy, no agent has been found to prevent or improve peripheral neuropathy [8–11], except duloxetine which has promising effects [12]. Another treatment strategy of oxaliplatin-containing therapy, the oxaliplatin stop-and-go strategy, was studied in the OPTIMOX1 trial [13]. This strategy comprised a sequence of an induction therapy, a maintenance therapy, oxaliplatin withdrawal, and a possible oxaliplatin reintroduction. Infusional 5-fluorouracil/folinic acid (LV5FU) combined with oxaliplatin (FOLFOX), followed by LV5FU and later oxaliplatin reintroduction showed a favorable disease control duration and decreased the occurrence of peripheral neuropathy in patients with mCRC, compared to FOLFOX alone which continued until disease progression. Several studies have reported various durations of induction therapy and various maintenance therapy regimens including a chemotherapy-free interval with or without oxaliplatin reintroduction. In a meta-analysis, such intermittent strategies in patients with mCRC showed no clinically significant reduction in overall survival (OS) when compared with a continuous strategy [14]. However, the optimal maintenance treatment strategy remains unclear.
In this study, we aimed to investigate the optimal duration of both induction and maintenance therapy regimens, including the necessity of oxaliplatin reintroduction as first-line oxaliplatin-containing therapy in patients with mCRC, using a trial-level meta-analysis.
This study was registered in the PROSPERO database (CRD42015019077) and was conducted according to the Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) statement [15]. Trials involving mCRC that were published up to March 2018 were identified through a systematic search of the PubMed database using the keywords [“colorectal cancer” (All Fields) OR “colorectal cancers” (All Fields) OR “colorectal neoplasm” (All Fields) OR “colorectal neoplasms” (All Fields)] AND [“maintenance” (All Fields) OR “stop and go” (All Fields) OR “no treatment” (All Fields) OR “holiday” (All Fields) OR “reintroduction” (All Fields) OR “intermittent” (All Fields) OR “OPTIMOX” (All Fields)] OR “oxaliplatin” (All Fields) OR “XELOX” (All Fields) OR “CAPOX” (All Fields) OR “FOLFOX” (All Fields)]. A manual search was also performed for abstracts presented at annual meetings of the American Society of Clinical Oncology, Gastrointestinal Cancers Symposium, European Society of Medical Oncology, and World Congress of Gastrointestinal Cancer up to March 2018.
Inclusion criteria were formed using the participants, intervention, control, outcomes, and study design (PICOS) strategy as follows: P (participants), mCRC patients; I (intervention), maintenance strategy for oxaliplatin-containing therapy as first-line treatment; C (control), no control; O (outcomes), the OS from induction therapy; and S (study design), phase II and III trials. The following studies were excluded: anti-EGFR antibody-containing induction therapy; no information on duration of induction therapy; no details on the maintenance therapy, oxaliplatin reintroduction, or OS; combination therapy with irinotecan; combination therapy with targeted agents, except bevacizumab; maintenance therapy with a drug that had not been used for induction therapy; a specific group of patients, such as the elderly (described in the title or text as targeting elderly or older patients) or patients with only liver metastasis; and non-English reports. In trials where patients were randomized at the start of maintenance therapy, the duration of induction therapy when predefined in the protocol was added to the reported median OS.
Two authors (T.M., A.S.) independently extracted information from the selected literature using predefined data extraction forms. The following details were extracted: the year of publication or presentation, number of patients enrolled or analyzed, primary endpoint, induction therapy regimen, and maintenance therapy regimen with or without oxaliplatin reintroduction. The following information was collected: the trial start year, median OS, median progression-free survival (PFS), defined duration of induction and maintenance therapies, and proportion of patients who underwent maintenance therapy and oxaliplatin reintroduction. The induction therapy regimens were classified as combination with and without bevacizumab. Maintenance therapy regimens were classified as no treatment (supportive care only), FP/bevacizumab alone, and FP plus bevacizumab.
Our primary endpoint was the OS. There were four exploratory variables: the induction therapy regimen, duration of induction therapy, maintenance therapy regimen, and oxaliplatin reintroduction rate. These variables were necessary to construct the model for the oxaliplatin stop-and-go strategy. In addition, two variables, the maintenance therapy rate (defined as the proportion of patients who switched from the induction therapy to the maintenance therapy) and trial start year, were used to adjust for in the analysis as affecting factors of the OS. The relationships between the median OS and these variables were assessed using correlation analysis and weighted multivariate regression analysis. Spearman’s partial correlation coefficients (pR) were used to evaluate the correlations between the median OS and these variables. Pearson’s pR were used in sensitivity analyses of Spearman’s pR. Median OS was predicted using the multiple regression model with these variables weighted according to the sample size of the treatment arms. An OS prediction model was developed using interaction terms between each variable (the induction therapy regimen, duration of induction therapy, and maintenance therapy regimen) that was adjusted. Variables with the largest p-values of interaction were excluded from the model, and the analysis was repeated until the p-value of all the interacting terms was less than 0.1. Variables related to significant interaction terms were finally included in the model. The adaptability of the model was evaluated using the coefficient of determination and adjusted for the degrees of freedom (adjusted R2). We considered that the model was well fitted if the adjusted R2 was above 0.50. To establish the validity and generalizability of the prediction model, the model was applied to some extra trials to evaluate the discrepancy between the predicted and observed OS. Treatment arms, which were excluded from this analysis because of the lack of information on the maintenance therapy rate, were included in the extra-validation set. The maintenance therapy rate was assumed to be the weighted mean maintenance therapy rate among trials in the original set. As exploratory analysis, the median PFS was analyzed using similar methods used for the OS. The relationship between the median PFS and variables (except oxaliplatin reintroduction rate) were assessed in the treatment arms which continued the maintenance therapy until disease progression. The maintenance therapy rate was used to adjust for in the analysis as an affecting factor of PFS. Statistical tests were two-sided with a 5% significance level. Analyses were performed using SAS software ver. 9.4 (SAS Institute Inc., Cary, NC).
Among the 4,551 studies retrieved up to March 2018, 22 treatment arms in 15 trials were identified; we included 2,581 patients in the analysis (Fig. 1) [16–30]. Characteristics of the treatment arm are summarized in Table 1 (details of the respective treatment arms are listed in S1 Table). Uracil-tegafur as maintenance therapy after FOLFOX was adopted in one treatment arm [17]; this was included in our meta-analysis because it is an FP-based drug. Regarding the two treatment arms in which the duration of induction therapy was defined as 18 to 24 weeks, 18 weeks was adopted because 88% of patients received an induction therapy of 18 weeks [28]. Eighteen treatment arms were recommended to continue maintenance therapy until disease progression. Oxaliplatin reintroduction was predefined in 15 treatment arms. In 11 treatment arms, oxaliplatin reintroduction was recommended after failure of maintenance therapy. In another four treatment arms, it was recommended after a fixed maintenance therapy period or after an earlier disease progression during maintenance therapy. In all of them, the duration of oxaliplatin reintroduction therapy was until disease progression. The median oxaliplatin reintroduction rate was 31.0% (range, 0.0% to 76.9%). Although the study began with a maintenance therapy in five treatment arms, the median OS from the induction therapy was reported in only two treatment arms.
Correlations between the OS and the variables examined are listed in S2 Table and shown in Fig. 2. The maintenance therapy regimen showed the highest correlation with the OS (Spearman’s pR=0.42). The induction therapy regimen, duration of induction therapy, and oxaliplatin reintroduction rate weakly correlated with the OS (pR=0.09, pR=0.06, and pR=0.15, respectively). In the multivariate regression analysis for OS, there was no significant association with any variable except with the maintenance therapy rate (S3 Table). According to the multiple regression model including interaction terms, the maintenance therapy regimen and rate were significantly associated with the OS (p=0.007 and p < 0.001, respectively), and a significant interaction term was observed between the duration of induction therapy and maintenance therapy regimen (p=0.019) (Table 2). Based on the results of the multiple regression analysis including the interaction term, an OS prediction model was built using the duration of induction therapy, maintenance therapy rate, and maintenance therapy regimen (adjusted R2=0.80). Thus, the OS prediction model was given as
where X1 is the induction therapy (weeks), X21=1 for the maintenance therapy with FP/bevacizumab alone and X21=0 for others, X22=1 for the maintenance therapy with FP+bevacizumab and X22=0 for the others, and X3 is the maintenance therapy rate (10%).
Ten treatment arms were included in the extra-validation set (Fig. 3, S4 Table) [13,31–37]. The temporary maintenance therapy rate was 74%. The observed median OS in most extra-validation treatment arms was included within the 95% confidence interval (CI) of the predicted median OS, except for three treatment arms.
Based on the assumption that a patient received maintenance therapy after induction therapy, the lines to predict the median OS were derived by applying the duration of induction therapy of 12 to 27 weeks and the maintenance therapy regimens (Fig. 4). When FP plus bevacizumab was used in the maintenance therapy, the longest and shortest predicted median OS were 28.6 months (95% CI, 25.6 to 31.7) at 12 weeks and 24.2 months (95% CI, 20.7 to 27.6) at 27 weeks, respectively. In contrast, the predicted OS for maintenance therapy with no treatment or with FP or bevacizumab became longer as the duration of induction therapy was prolonged. The shortest predicted median OS was 20.5 months (95% CI, 17.7 to 23.3) and 25.9 months (95% CI, 22.8 to 29.0) for maintenance therapy with no treatment or with FP or bevacizumab, respectively, at 12 weeks. The longest predicted median OS was 27.5 months (95% CI, 24.4 to 30.5) and 28.8 months (95% CI, 26.0 to 31.6) for maintenance therapy with no treatment or with FP or bevacizumab, respectively, at 27 weeks. The predicted median OS for maintenance therapy with FP plus bevacizumab crossed the line for that with FP or bevacizumab between 17 and 18 weeks of induction therapy, whereas that for maintenance therapy with no treatment crossed between 22 and 23 weeks.
Correlations between the PFS and the variables examined are listed in S2 Table and shown in Fig. 2. The maintenance therapy regimen showed the highest correlation with PFS (Spearman’s pR=0.82). The induction therapy regimen and duration of induction therapy weakly correlated with the PFS (pR=0.15 and pR=−0.03, respectively). In the multivariate regression analysis for PFS, it was significantly associated with the maintenance therapy regimen and maintenance therapy rate (S3 Table). Finally, the maintenance therapy regimen and rate were selected, and no significant interaction term was observed (Table 2). Based on the results, the PFS prediction model was given as
where X11=1 for the maintenance therapy with FP/bevacizumab alone and X11=0 for the others, X12=1 for maintenance therapy with FP+bevacizumab and X12=0 for the others, and X2 is the maintenance therapy rate (10%). The adjusted R2 of the model was 0.83.
Eight treatment arms were included in the extra-validation set (Fig. 3, S4 Table) [31–35,37]. The observed median PFS in all extra-validation treatment arms was included within 95% CI of the predicted median PFS. The predicted PFS was calculated based on the assumption that a patient received maintenance therapy after an induction therapy (Fig. 4). The predicted median PFS for maintenance therapy with no treatment, with FP or bevacizumab, and with FP plus bevacizumab was 8.4 months (95% CI, 7.6 to 9.2), 9.9 months (95% CI, 8.9 to 11.0), and 12.2 months (95% CI, 11.2 to 13.2), respectively.
We studied the optimal maintenance strategy of oxaliplatin-containing therapy in patients with mCRC in this trial-level meta-analysis and found that the maintenance therapy regimen correlated highest with the OS, while the oxaliplatin reintroduction rate weakly correlated with OS. Moreover, we were able to build an OS prediction model with high accuracy.
Although the maintenance therapy regimen showed the highest correlation with OS in our meta-analysis, the impact of the duration of induction therapy should also be considered. The predicted OS on maintenance therapy with FP plus bevacizumab was superior to that with FP or bevacizumab for up to 17 weeks of induction therapy. This boundary week (17 to 18 weeks from the start of induction therapy) is known to be the usual time when grade 2 or more oxaliplatin-related peripheral neuropathy develops [38,39]. Considering that most patients would have discontinued oxaliplatin by that time, it is better that patients who have grade 2 or more oxaliplatin-related peripheral neuropathy until 17 weeks of induction therapy switch from induction therapy to maintenance therapy with FP plus bevacizumab as soon as possible. In contrast, some patients who can continue oxaliplatin for a long time without related toxicities might be able to obtain survival benefits with the continuation of induction therapy. According to our OS prediction model, maintenance therapy with FP or bevacizumab was adequate when the duration of induction therapy was over 18 weeks. Patients who have grade 1 or less oxaliplatin-related toxicities including long-standing peripheral neuropathy should continue the induction therapy for as long as possible.
When oxaliplatin is discontinued despite being effective, its sensitivity in such patients is maintained. The oxaliplatin reintroduction rate correlated with a prolonged OS in a retrospective analysis of the OPTIMOX1 trial [40]. A previous meta-analysis of alternative endpoints to evaluate a therapeutic strategy comprising oxaliplatin reintroduction in mCRC showed a good correlation between the time to failure of treatment strategy and OS. However, this meta-analysis was conducted in only three randomized trials including the OPTIMOX1 trial, which had the largest number of patients [41]. The duration of maintenance therapy was 12 weeks in this trial and not until disease progression. This means that the sensitivity to FP was maintained in many patients. In most trials, including our meta-analysis, maintenance therapy was continued until disease progression, and the oxaliplatin reintroduction rate correlated moderately with the time to failure of treatment strategy (pR=0.32; data not shown), but weakly with the OS (pR=0.15). Therefore, most patients failed to achieve survival benefits from oxaliplatin reintroduction. It might also be important that patients are sensitive to FP when oxaliplatin reintroduction is being considered. Our meta-analysis could not answer this hypothesis because we used only four corresponding treatment arms.
Although an OS prediction model with a high accuracy was built, it did not include the observed median OS within the predicted OS in three extra-validation treatment arms. The OS with chronomodulated capecitabine plus oxaliplatin was shorter than that with the standard treatment schedule in a study by Qvortrup et al. [32]. In half of the patients who received capecitabine plus oxaliplatin combined with bevacizumab, the regimen was discontinued because of reasons other than disease progression as reported by Tol et al. [31] and Diaz-Rubio et al. [35]. These reasons might not apply to our OS prediction model.
We exploratory analyzed the relationship between the PFS and related variables. PFS was analyzed among trials recommended to continue maintenance therapy until disease progression, since the definition of PFS differed between trials recommended to continue maintenance therapy until disease progression and trials with fixed maintenance therapy periods, even if no disease progression was observed during the maintenance therapy. The maintenance therapy regimen showed the highest correlation with the PFS, and a PFS prediction model with high accuracy was built. The importance of maintenance therapy with FP plus bevacizumab in the analysis of the PFS also supports our results for the OS. According to this predicted PFS model, the PFS was longest in the maintenance therapy with FP plus bevacizumab, regardless of the duration of induction therapy. Therefore, switching to this maintenance therapy early might not be disadvantageous to the patients.
This meta-analysis had several limitations. First, this study was not analyzed based on individual patient data, and the correlation between efficacy outcomes and oxaliplatin-related adverse events could not be identified. Second, three treatment arms had information only on the OS from the start of maintenance therapy. The accuracy might be decreased in those treatment arms because the defined duration of induction therapy was added. Third, although a maintenance strategy was conducted in anti-EGFR antibody-containing induction therapy studies, these studies were not included in this meta-analysis because they involved analysis of patients solely with wild-type KRAS/NRAS. Fourth, not all trials z included in the analysis had an OS as the primary endpoint. However, the event of death might suffice because the median follow-up time in these trials was 36.2 months as the median OS from first-line chemotherapy has reached 30 months in recent years [7]. Last, treatment arms on maintenance therapy with a drug that was not used in the induction therapy, called the switch maintenance strategy, were excluded. This strategy is not common in patients with mCRC but has been proven effective in patients with lung cancer [42].
In conclusion, the maintenance therapy regimen showed the strongest correlation with median OS, and the correlation between the OS and oxaliplatin reintroduction was weak. This meta-analysis suggests that in most patients with mCRC, the optimal maintenance strategy of oxaliplatin-containing therapy is an induction therapy of up to 17 weeks (approximately 4 months) followed by a maintenance therapy with FP plus bevacizumab. In patients with mild oxaliplatin-induced toxicities, the induction therapy should be continued for as long as possible, and FP or bevacizumab alone is adequate as maintenance therapy. Further randomized trials to study the duration of induction therapy and oxaliplatin reintroduction are needed.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Author Contributions
Conceived and designed the analysis: Moriwaki T, Gosho M, Sugaya A, Yamada T, Yamamoto Y, Hyodo I.
Collected the data: Moriwaki T, Sugaya A.
Contributed data or analysis tools: Moriwaki T, Sugaya A.
Performed the analysis: Gosho M.
Wrote the paper: Moriwaki T, Gosho M, Sugaya A, Yamada T, Yamamoto Y, Hyodo I.
References
1. de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000; 18:2938–47.

2. Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006; 24:3347–53.

3. Porschen R, Arkenau HT, Kubicka S, Greil R, Seufferlein T, Freier W, et al. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO Colorectal Study Group. J Clin Oncol. 2007; 25:4217–23.

4. Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004; 22:229–37.

5. Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010; 28:4697–705.

6. Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008; 26:2013–9.

7. Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized cinical trial. JAMA. 2017; 317:2392–401.
8. Grothey A, Nikcevich DA, Sloan JA, Kugler JW, Silberstein PT, Dentchev T, et al. Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. J Clin Oncol. 2011; 29:421–7.

9. Hochster HS, Grothey A, Hart L, Rowland K, Ansari R, Alberts S, et al. Improved time to treatment failure with an intermittent oxaliplatin strategy: results of CONcePT. Ann Oncol. 2014; 25:1172–8.

10. Loprinzi CL, Qin R, Dakhil SR, Fehrenbacher L, Flynn KA, Atherton P, et al. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J Clin Oncol. 2014; 32:997–1005.

11. Oki E, Emi Y, Kojima H, Higashijima J, Kato T, Miyake Y, et al. Preventive effect of Goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): a placebo-controlled, double-blind, randomized phase III study. Int J Clin Oncol. 2015; 20:767–75.

12. Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013; 309:1359–67.
13. Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol. 2006; 24:394–400.

14. Berry SR, Cosby R, Asmis T, Chan K, Hammad N, Krzyzanowska MK, et al. Continuous versus intermittent chemotherapy strategies in metastatic colorectal cancer: a systematic review and meta-analysis. Ann Oncol. 2015; 26:477–85.

15. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009; 6:e1000100.

16. Andre T, Tournigand C, Mineur L, Fellague-Chebra R, Flesch M, Mabro M, et al. Phase II study of an optimized 5-fluorouracil-oxaliplatin strategy (OPTIMOX2) with celecoxib in metastatic colorectal cancer: a GERCOR study. Ann Oncol. 2007; 18:77–81.
17. Scalamogna R, Brugnatelli S, Tinelli C, Sagrada P, Gattoni E, Tronconi MC, et al. UFT as maintenance therapy in patients with advanced colorectal cancer responsive to the FOLFOX4 regimen. Oncology. 2007; 72:267–73.

18. Chibaudel B, Maindrault-Goebel F, Lledo G, Mineur L, Andre T, Bennamoun M, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol. 2009; 27:5727–33.

19. Comella P, Massidda B, Filippelli G, Farris A, Natale D, Barberis G, et al. Randomised trial comparing biweekly oxaliplatin plus oral capecitabine versus oxaliplatin plus i.v. bolus fluorouracil/leucovorin in metastatic colorectal cancer patients: results of the Southern Italy Cooperative Oncology study 0401. J Cancer Res Clin Oncol. 2009; 135:217–26.

20. Waddell T, Gollins S, Soe W, Valle J, Allen J, Bentley D, et al. Phase II study of short-course capecitabine plus oxaliplatin (XELOX) followed by maintenance capecitabine in advanced colorectal cancer: XelQuali study. Cancer Chemother Pharmacol. 2011; 67:1111–7.

21. Tezuka T, Hamada C, Ishida H, Ooshiro M, Matsuoka H, Kawasaki S, et al. Phase II clinical study of modified FOLFOX7 (intermittent oxaliplatin administration) plus bevacizumab in patients with unresectable metastatic colorectal cancer-CRAFT study. Invest New Drugs. 2013; 31:1321–9.

22. Yalcin S, Uslu R, Dane F, Yilmaz U, Zengin N, Buyukunal E, et al. Bevacizumab+capecitabine as maintenance therapy after initial bevacizumab+XELOX treatment in previously untreated patients with metastatic colorectal cancer: phase III ‘Stop and Go’ study results: a Turkish Oncology Group Trial. Oncology. 2013; 85:328–35.
23. Hong YS, Lee SS, Kim KP, Lee JL, Kang YK, Shin SJ, et al. A phase II study of bevacizumab, oxaliplatin, and capecitabine in patients with previously untreated metastatic colorectal cancer: a prospective, multicenter trial of the Korean Cancer Study Group. Am J Clin Oncol. 2014; 37:19–23.
24. Kim ST, Hong YS, Lim HY, Lee J, Kim TW, Kim KP, et al. S-1 plus oxaliplatin versus capecitabine plus oxaliplatin for the first-line treatment of patients with metastatic colorectal cancer: updated results from a phase 3 trial. BMC Cancer. 2014; 14:883.

25. Hegewisch-Becker S, Graeven U, Lerchenmuller CA, Killing B, Depenbusch R, Steffens CC, et al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2015; 16:1355–69.

26. Nakayama N, Sato A, Tanaka S, Shimada K, Konishi K, Sasaki E, et al. A phase II study of bevacizumab with modified OPTIMOX1 as first-line therapy for metastatic colorectal cancer: the TCOG-GI 0802 study. Invest New Drugs. 2015; 33:954–62.

27. Simkens LH, van Tinteren H, May A, ten Tije AJ, Creemers GJ, Loosveld OJ, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015; 385:1843–52.

28. Luo HY, Li YH, Wang W, Wang ZQ, Yuan X, Ma D, et al. Single-agent capecitabine as maintenance therapy after induction of XELOX (or FOLFOX) in first-line treatment of metastatic colorectal cancer: randomized clinical trial of efficacy and safety. Ann Oncol. 2016; 27:1074–81.

29. Nakayama G, Ishigure K, Yokoyama H, Uehara K, Kojima H, Ishiyama A, et al. The efficacy and safety of CapeOX plus bevacizumab therapy followed by capecitabine plus bevacizumab maintenance therapy in patients with metastatic colorectal cancer: a multi-center, single-arm, phase II study (CCOG-0902). BMC Cancer. 2017; 17:243.

30. Kosugi C, Koda K, Denda T, Ishibashi K, Ishida H, Seike K, et al. VOICE trial: Final results from multicenter phase II study of assessment of clinical efficiency and safety in capecitabine plus intermittent oxaliplatin together with bevacizumab as first-line therapy for patients with advanced colorectal cancer. J Clin Oncol. 2018; 36(4 Suppl):740.
31. Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009; 360:563–72.

32. Qvortrup C, Jensen BV, Fokstuen T, Nielsen SE, Keldsen N, Glimelius B, et al. A randomized study comparing short-time infusion of oxaliplatin in combination with capecitabine XELOX(30) and chronomodulated XELOX(30) as first-line therapy in patients with advanced colorectal cancer. Ann Oncol. 2010; 21:87–91.

33. Vaidyanathan G, Groman A, Wilding G, Fakih MG. Stop and go FOLFOX plus bevacizumab chemotherapy in the first-line treatment of metastatic colorectal cancer. Oncology. 2010; 79:67–71.

34. Ducreux M, Bennouna J, Hebbar M, Ychou M, Lledo G, Conroy T, et al. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line treatment for metastatic colorectal cancer. Int J Cancer. 2011; 128:682–90.

35. Diaz-Rubio E, Gomez-Espana A, Massuti B, Sastre J, Abad A, Valladares M, et al. First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD study. Oncologist. 2012; 17:15–25.
36. Okita NT, Esaki T, Baba E, Sakai D, Tokunaga S, Takiuchi H, et al. A multicenter phase II study of the stop-and-go modified FOLFOX6 with bevacizumab for first-line treatment of patients with metastatic colorectal cancer. Invest New Drugs. 2012; 30:2026–31.

37. Antonuzzo L, Giommoni E, Pastorelli D, Latiano T, Pavese I, Azzarello D, et al. Bevacizumab plus XELOX as first-line treatment of metastatic colorectal cancer: The OBELIX study. World J Gastroenterol. 2015; 21:7281–8.

38. Grothey A. Clinical management of oxaliplatin-associated neurotoxicity. Clin Colorectal Cancer. 2005; 5(Suppl 1):S38–46.

39. Beijers AJ, Mols F, Vreugdenhil G. A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer. 2014; 22:1999–2007.

40. de Gramont A, Buyse M, Abrahantes JC, Burzykowski T, Quinaux E, Cervantes A, et al. Reintroduction of oxaliplatin is associated with improved survival in advanced colorectal cancer. J Clin Oncol. 2007; 25:3224–9.

41. Chibaudel B, Bonnetain F, Shi Q, Buyse M, Tournigand C, Sargent DJ, et al. Alternative end points to evaluate a therapeutic strategy in advanced colorectal cancer: evaluation of progression-free survival, duration of disease control, and time to failure of strategy: an Aide et Recherche en Cancerologie Digestive Group Study. J Clin Oncol. 2011; 29:4199–204.
Fig. 1
Study selection according to the PRISMA diagram. ASCO, American Society of Clinical Oncology; ESMO, European Society of Medical Oncology; PRISMA, Preferred Reporting Items for Systemic Reviews.
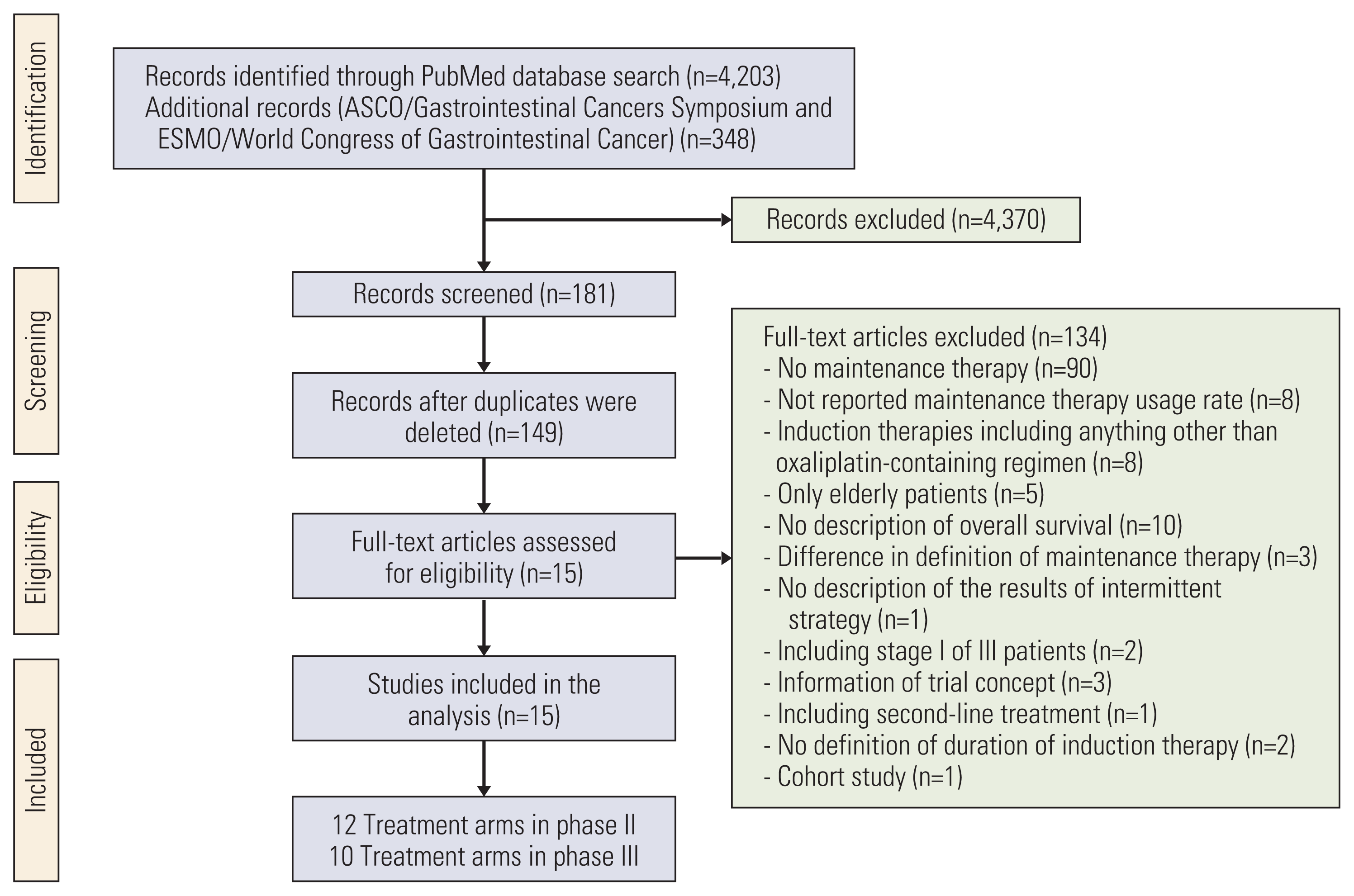
Fig. 2
Correlations between the overall survival or progression-free survival and variables: induction therapy regimen (A, B), duration of induction therapy (C, D), maintenance therapy regimen (E, F), and oxaliplatin reintroduction rate (G). The size of each circle is proportional to the sample size. The solid line indicates the estimated regression line, and the dotted line indicates the 95% confidence interval. Bev, bevacizumab; FP, fluoropyrimidine; pR, Spearman’s partial correlation coefficient; R, Spearman’s correlation coefficient.
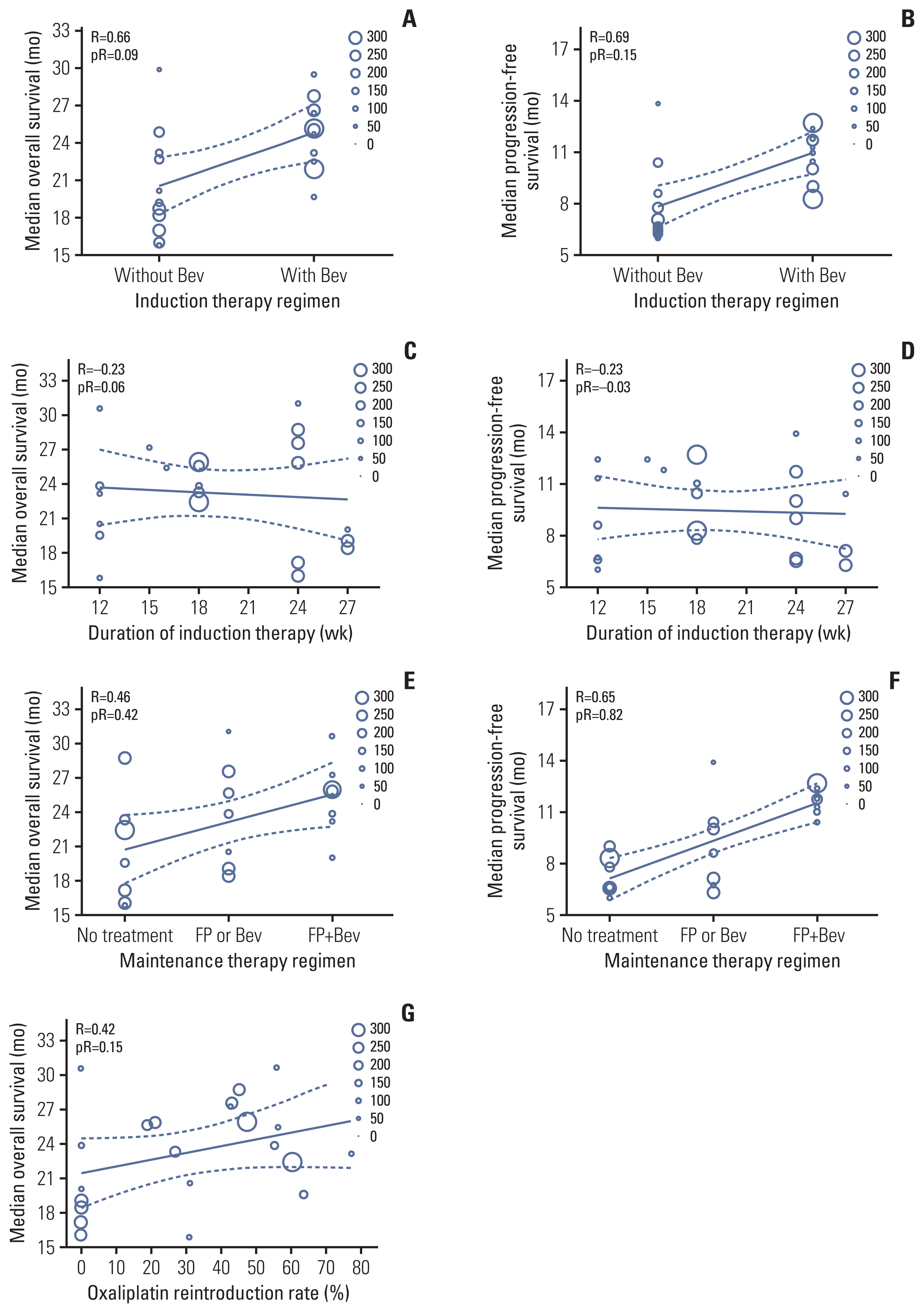
Fig. 3
Relationships between observed and predicted survival times in the extra-validation treatment arms: overall survival (A) and progression-free survival (B). Predicted progression-free survival was calculated in treatment arms recommended to continue maintenance therapy until disease progression. Bev, bevacizumab; CAPOX, capecitabine and oxaliplatin; CI, confidence interval; FOLFOX, infusional 5-fluorouracil, folinic acid, and oxaliplatin; mFOLFOX, modified infusional 5-fluorouracil, folinic acid, and oxaliplatin.
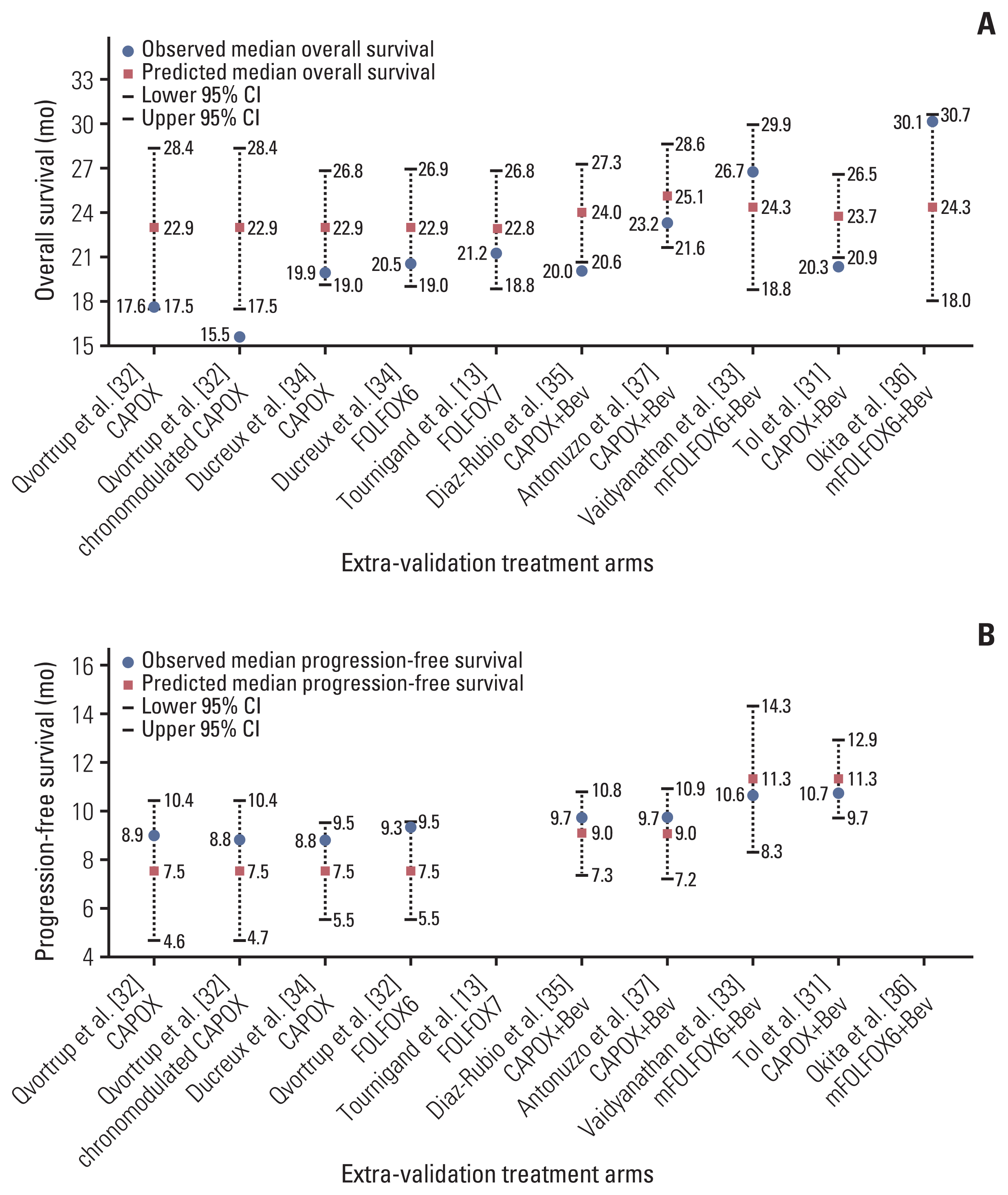
Fig. 4
Relationships between the predicted survival time and duration of induction therapy, and maintenance therapy regimen, with maintenance therapy rate of 100%: overall survival (A) and progression-free survival (B). CI, confidence interval; FP, fluoropyrimidine.
Table 1
Characteristics of 22 treatment arms in 15 trials
| No. (%) (n=2,581) | |
|---|---|
| Patients per treatment arm | 120 (30–279) |
| Phase | |
| II | 12 treatment arms |
| III | 10 treatment arms |
| Trial start year (yr) | 2008 (2002–2011) |
| Induction therapy | |
| Regimen | |
| Cytotoxic drugs alone | 11 |
| Cytotoxic drugs+bevacizumab | 11 |
| Duration of treatment (wk) | |
| 12 | 6 |
| 15 | 1 |
| 16 | 1 |
| 18 | 3 |
| 24 | 6 |
| 27 | 3 |
| 18–24 | 2 |
| Maintenance therapy | |
| Rate | 80.6 (11.6–100) |
| 66.7 (11.6–90.7)a) | |
| Regimen | |
| No treatment | 7 |
| Fluoropyrimidine alone | 6b) |
| Bevacizumab alone | 1 |
| Fluoropyrimidine+bevacizumab | 8 |
| Duration of treatment | |
| Until disease progression | 18 |
| 15 wk | 1 |
| 16 wk | 1 |
| 24 wk | 2 |
| Oxaliplatin reintroduction therapy | |
| Recommended by protocol | |
| Yes | 15 |
| Rate | 31.0 (0.0–76.9) |
| 45.0 (19.1–76.9)c) | |
| Follow-up time (mo) | 36.2 (9.3–60.0)d) |
| Overall survival (mo) | 23.6 (15.8–31.0) |
| Progression-free survival (mo) | 9.5 (6.0–13.9) |
| 8.5 (6.0–13.9)e) | |
Table 2
Multivariate regression analysis with interaction terms
| Outcome | Independent variable | Unit | Analysis of variance | Multivariate regression analysis | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| F | p-value | Regression coefficient (95% CI) | p-value | |||
| Overall survival | Duration of induction therapya) | 1-week | 1.61 | 0.220 | 0.46 (0.13 to 0.80) | 0.009 |
|
|
||||||
| Maintenance therapy regimen | FP or Bev | 7.18 | 0.007 | 8.72 (−0.07 to 17.50) | 0.052 | |
|
|
||||||
| Control: no treatment | FP+Bev | 17.31 (7.53 to 27.09) | 0.002 | |||
|
|
||||||
| Maintenance therapy rate | 10% | 63.8 | < 0.001 | 1.20 (−0.88 to 1.52) | < 0.001 | |
|
|
||||||
| Interaction between duration of induction therapy and maintenance therapy regimen | 1-week×FP or Bev | 5.27 | 0.019 | −0.27 (−0.68 to 0.14) | 0.180 | |
|
|
||||||
| 1-week×FP+Bev | −0.76 (−1.26 to −0.26) | 0.005 | ||||
|
|
||||||
| Progression-free survival | Maintenance therapy regimen | FP or Bev | 24.2 | < 0.001 | 1.52 (0.44 to 2.60) | 0.009 |
|
|
||||||
| Control: no treatment | FP+Bev | 3.84 (2.66 to 5.02) | < 0.001 | |||
|
|
||||||
| Maintenance therapy rate | 10% | 24.7 | < 0.001 | 0.34 (0.20 to 0.49) | < 0.001 | |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download