Abstract
Purpose
Materials and Methods
Results
Notes
Ethical Statement
This study was performed with the approval of the Institutional Review Board (IRB) of Asan Medical Center (IRB Approval No. 2020-0153). Obtaining informed consent was waived by the IRB, since this study was retrospective medical records review study.
References
Fig. 1
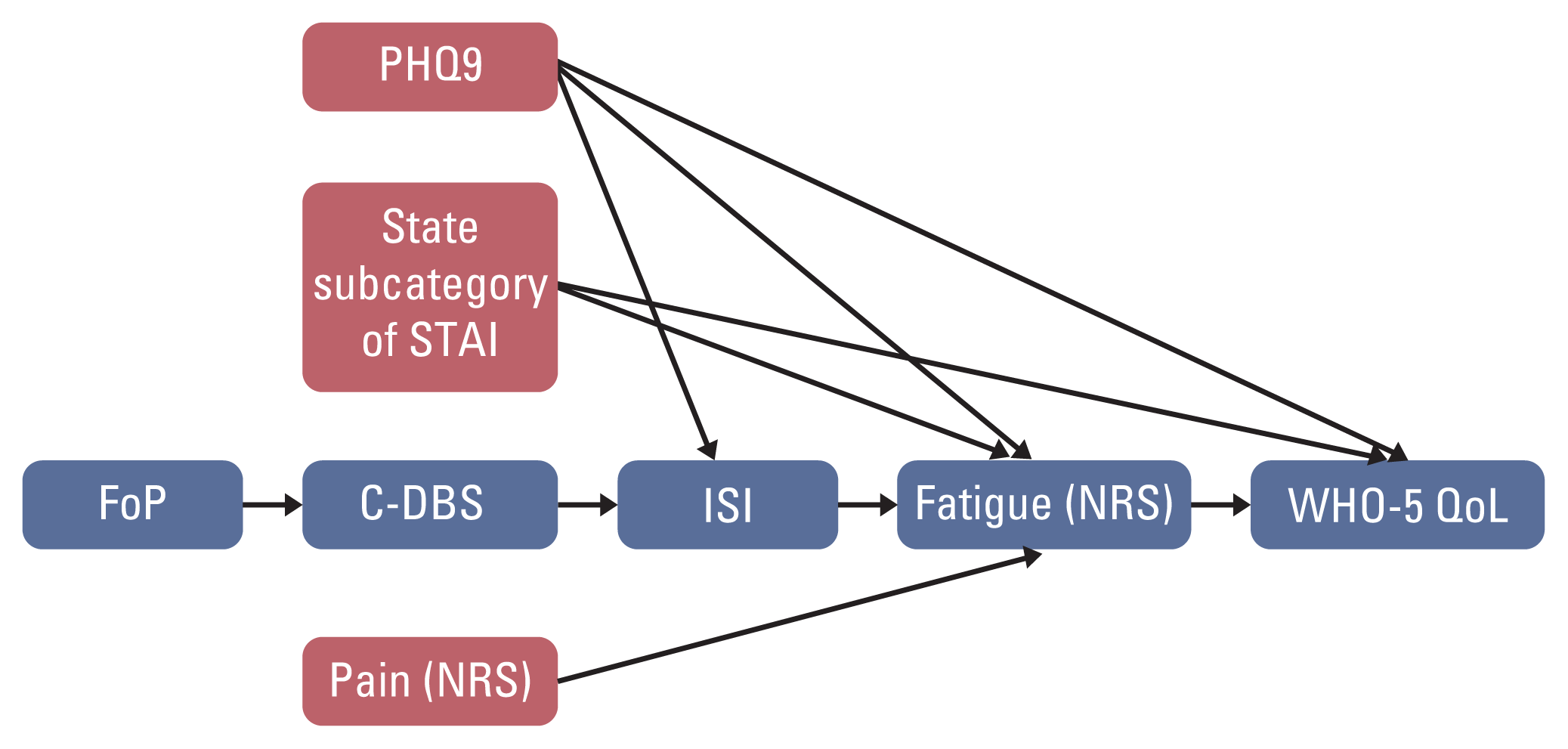
Fig. 2
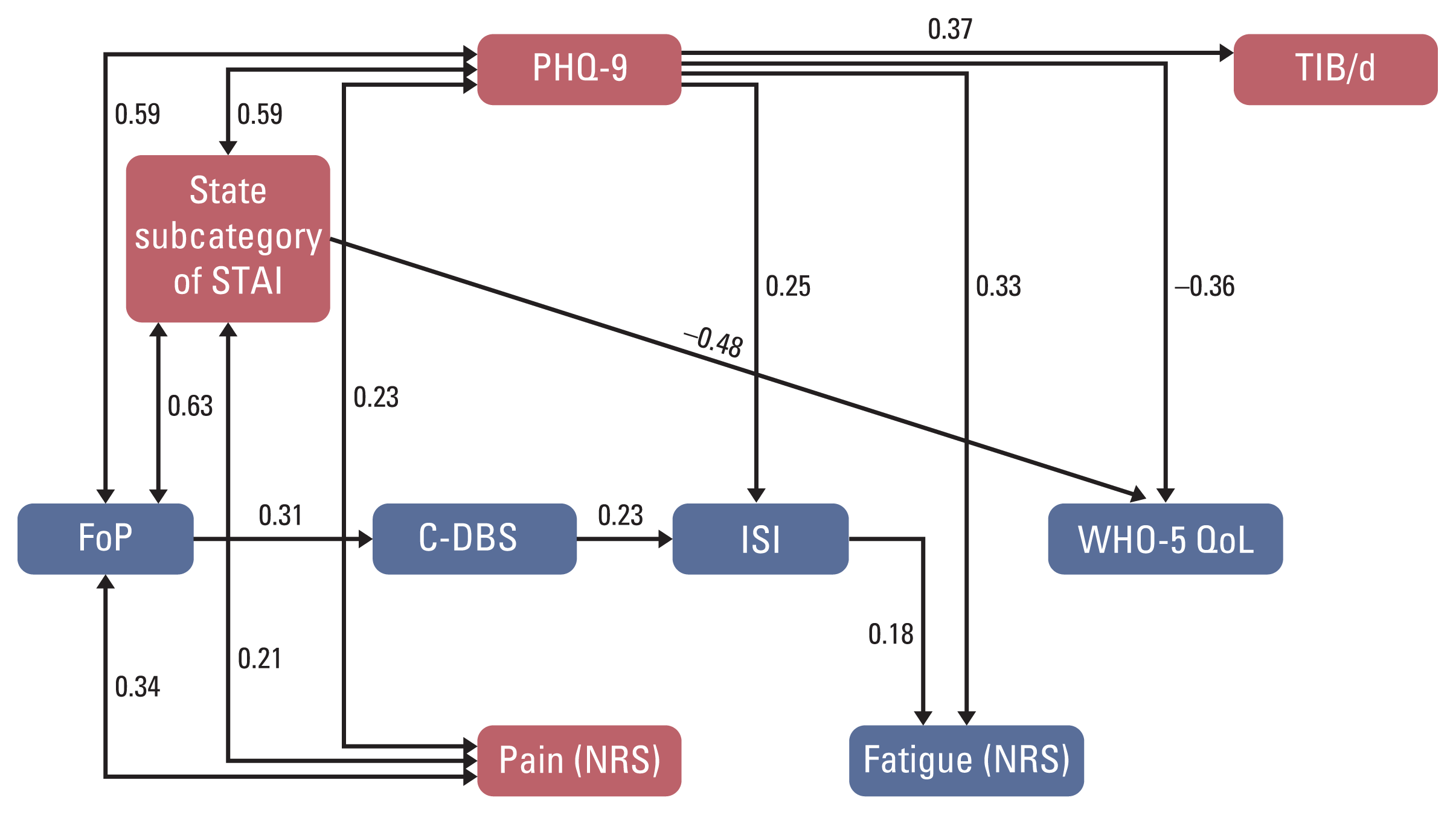
Table 1
Table 2
| Variable | Age | WHO-5 | PHQ-9 | ISI | STAI-S | FoP | C-DBS | Pain | Fatigue | TIB/d |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1.000 | |||||||||
| WHO-5 | 0.043 | 1.000 | ||||||||
| PHQ-9 | −0.172* | −0.648** | 1.000 | |||||||
| ISI | −0.030 | −0.179* | 0.405** | 1.000 | ||||||
| STAI-S | −0.161* | −0.694** | 0.590** | 0.171* | 1.000 | |||||
| FoP | −0.402** | −0.420** | 0.565** | 0.194* | 0.571** | 1.000 | ||||
| C-DBS | −0.140 | −0.172* | 0.290** | 0.330** | 0.195* | 0.277** | 1.000 | |||
| Pain | −0.090 | −0.163* | 0.282* | 0.171* | 0.231** | 0.342** | 0.246** | 1.000 | ||
| Fatigue | −0.208** | −0.350** | 0.480** | 0.358** | 0.327** | 0.308** | 0.251** | 0.288** | 1.000 | |
| TIB/d | −0.121 | −0.211* | 0.311** | 0.059 | 0.221** | 0.189* | 0.043 | 0.163 | 0.081 | 1.000 |
C-DBS, Cancer-Related Dysfunctional Beliefs about Sleep; FoP, Fear of Progression; ISI, Insomnia Severity Index; PHQ-9, Patient Health Questionnaire-9; STAI-S, State subcategory of State-Trait Anxiety Inventory; TIB/d, Time in Bed during 24 hours, WHO-5, World Health Organization-5 well-being index.
Table 3
| Variable | WHO-5 top 75% (n=114) | WHO-5 bottom 25% (n=45) | p-value |
|---|---|---|---|
| Cancer stage | |||
| Stage I | 35 (30.7) | 10 (22.2) | 0.027* |
| Stage II | 32 (28.1) | 9 (20.0) | |
| Stage III | 14 (12.3) | 3 (6.7) | |
| Stage IV | 21 (18.4) | 9 (20.0) | |
| Not yet confirmed | 12 (10.5) | 14 (31.1) | |
| Stage IV vs. Others | 21 (18.4)/93 (81.6) | 9 (20.0)/36 (80.0) | 0.819 |
| History of cancer treatment | |||
| Radiotherapy | 11 (9.6) | 2 (4.4) | 0.231 |
| Chemotherapy | 29 (25.4) | 16 (35.6) | 0.202 |
| Hormone therapy | 39 (34.2) | 9 (20.0) | 0.079 |
| Immune therapy | 6 (5.3) | 3 (6.7) | 0.494 |
| Operation within 3 mo | 28 (24.6) | 12 (26.7) | 0.783 |
| History of hormone therapy | |||
| Breast cancer with radiotherapy | 37 (32.5) | 8 (17.8) | 0.124 |
| Breast cancer without radiotherapy | 29 (25.4) | 11 (24.4) | |
| Others | 48 (42.1) | 26 (57.8) | |
| Cancer-related fatigue | |||
| High | 28 (24.6) | 20 (44.4) | 0.014* |
| Low | 86 (75.4) | 25 (55.6) | |
| Time in bed during daytime (min) | |||
| ≤ 30 vs. > 30 | 45 (45.5)/54 (54.5) | 10 (25.0)/30 (75.0) | 0.026* |
| ≤ 60 vs. > 60 | 57 (57.6)/42 (42.4) | 16 (40.0)/24 (60.0) | 0.060 |
| ≤ 120 vs. > 120 | 69 (69.7)/30 (30.3) | 20 (50.0)/20 (50.0) | 0.028* |
| Age (yr) | 55.0±11.9 | 54.4±10.8 | 0.786 |
| Sex | |||
| Male | 21 (18.4) | 8 (17.8) | 0.925 |
| Female | 93 (81.6) | 37 (82.2) | |
| Rating scale scores | |||
| ISI | 16.4±6.4 | 16.0±7.4 | 0.773 |
| PHQ-9 | 9.3±6.0 | 16.5±6.2 | < 0.001* |
| STAI-S | 38.5±10.4 | 52.0±9.6 | < 0.001* |
| FoP | 32.0±11.6 | 40.6±12.9 | < 0.001* |
| WHO-5 Questionnaire | 38.2±19.6 | 6.1±4.9 | < 0.001* |
| C-DBS | 11.3±4.9 | 12.6±5.9 | 0.141 |
| Pain (Numeric Rating Scale) | 2.8±2.5 | 3.0±2.6 | 0.548 |
| Fatigue (Numeric Rating Scale) | 5.6±2.4 | 6.6±2.7 | 0.040* |
| Sleep parameters | |||
| Bedtime | 10.6±1.0 | 10.9±1.2 | 0.296 |
| Sleep onset time | 11.8±1.7 | 12.0±1.6 | 0.481 |
| Wake up time | 6.8±1.3 | 7.0±1.6 | 0.289 |
| Sleep onset latency | 1.3±1.2 | 1.2±1.2 | 0.731 |
| Time in bed | 8.1±1.6 | 8.2±1.4 | 0.814 |
| Time in bed during 24 hr | 10.2±3.5 | 12.0±4.1 | 0.019* |
| Time in bed during daytime | 2.1±2.8 | 3.6±3.8 | 0.021* |
Values are presented as number (%) or mean±standard deviation. C-DBS, Cancer-Related Dysfunctional beliefs about Sleep; FoP, Fear of Progression; ISI, Insomnia Severity Index; PHQ-9, Patient Health Questionnaire-9; STAI-S, State subcategory of State-Trait Anxiety Inventory; WHO-5, World Health Organization-Five Well-Being Index.
Table 4
| Variable | Unadjusted | Age, sex adjusted | Multivariablea) adjusted | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age | 1.00 (0.97–1.03) | 0.784 | ||||
|
|
||||||
| Sex | 1.04 (0.42–2.57) | 0.925 | ||||
|
|
||||||
| History of cancer treatment | ||||||
|
|
||||||
| Radiotherapy | 0.44 (0.09–2.05) | 0.293 | 0.44 (0.09–2.09) | 0.301 | 0.45 (0.09–2.31) | 0.340 |
|
|
||||||
| Chemotherapy | 1.62 (0.77–3.40) | 0.204 | 1.68 (0.78–3.61) | 0.182 | 1.44 (0.61–3.39) | 0.408 |
|
|
||||||
| Hormone therapy | 0.48 (0.21–1.10) | 0.083 | 0.43 (0.18–1.02) | 0.057 | 0.70 (0.25–1.94) | 0.490 |
|
|
||||||
| Immune therapy | 1.29 (0.31–5.38) | 0.731 | 1.27 (0.30–5.33) | 0.747 | 1.56 (0.35–7.06) | 0.561 |
|
|
||||||
| Operation within 3 mo | 1.12 (0.51–2.45) | 0.783 | 1.11 (0.50–2.45) | 0.796 | 1.21 (0.50–2.93) | 0.673 |
|
|
||||||
| Cancer stage IV | 1.11 (0.46–2.64) | 0.819 | 1.11 (0.44–2.78) | 0.820 | 0.70 (0.23–2.11) | 0.530 |
|
|
||||||
| Rating scale scores | ||||||
|
|
||||||
| ISI | 0.99 (0.94–1.05) | 0.772 | 0.99 (0.94–1.05) | 0.777 | 0.99 (0.93–1.05) | 0.715 |
|
|
||||||
| PHQ–9 | 1.19 (1.12–1.27) | < 0.001 | 1.19 (1.12–1.28) | < 0.001 | 1.22 (1.13–1.32) | < 0.001 |
|
|
||||||
| STAI–S | 1.15 (1.10–1.21) | < 0.001 | 1.17 (1.11–1.24) | < 0.001 | 1.19 (1.11–1.26) | < 0.001 |
|
|
||||||
| C–DBS | 1.05 (0.98–1.13) | 0.142 | 1.05 (0.98–1.13) | 0.148 | 1.06 (0.98–1.14) | 0.122 |
|
|
||||||
| FoP | 1.06 (1.03–1.09) | 0.000 | 1.07 (1.03–1.11) | 0.000 | 1.07 (1.03–1.11) | 0.000 |
|
|
||||||
|
|
||||||
| Pain (Numeric Rating Scale) | 1.04 (0.91–1.20) | 0.545 | 1.04 (0.91–1.20) | 0.557 | 1.03 (0.89–1.20) | 0.693 |
|
|
||||||
| Fatigue | 1.16 (1.01–1.34) | 0.042 | 1.16 (1.00–1.35) | 0.043 | 1.20 (1.02–1.41) | 0.027 |
|
|
||||||
| Sleep parameters | ||||||
|
|
||||||
| Time in bed during 24 hr | 1.17 (1.05–1.32) | 0.006 | 1.17 (1.04–1.31) | 0.008 | 1.15 (1.01–1.31) | 0.037 |
|
|
||||||
| TIB daytime > 30 min | 3.06 (1.27–7.33) | 0.012 | 3.03 (1.26–7.28) | 0.014 | 2.84 (1.08–7.42) | 0.034 |
|
|
||||||
| TIB daytime > 60 min | 2.29 (1.06–4.94) | 0.036 | 2.26 (1.04–4.90) | 0.039 | 1.79 (0.76–4.22) | 0.181 |
|
|
||||||
| TIB daytime > 120 min | 2.52 (1.17–5.43) | 0.018 | 2.45 (1.13–5.32) | 0.023 | 1.80 (0.75–4.34) | 0.190 |
C-DBS, Cancer-Related Dysfunctional beliefs about Sleep; CI, confidence interval; FoP, Fear of Progression; ISI, Insomnia Severity Index; OR, odds ratio; PHQ-9, Patient Health Questionnaire-9; STAI-S, State subcategory of State-Trait Anxiety Inventory; TIB, time in bed; WHO-5, World Health Organization-Five Well-Being Index.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download