Abstract
Purpose
The purpose of this study was to investigate whether routine insertion of peripherally inserted central catheter (PICC) at admission to a hospice-palliative care (HPC) unit is acceptable in terms of safety and efficacy and whether it results in superior patient satisfaction compared to usual intravenous (IV) access.
Materials and Methods
Terminally ill cancer patients were randomly assigned to two arms: routine PICC access and usual IV access arm. The primary endpoint was IV maintenance success rate, defined as the rate of functional IV maintenance until the intended time (discharge, transfer, or death).
Results
A total of 66 terminally ill cancer patients were enrolled and randomized to study arms. Among them, 57 patients (routine PICC, 29; usual IV, 28) were analyzed. In the routine PICC arm, mean time to PICC was 0.84 days (range, 0 to 3 days), 27 patients maintained PICC with function until the intended time. In the usual IV arm, 11 patients maintained peripheral IV access until the intended time, and 15 patients underwent PICC insertion. The IV maintenance success rate in the routine PICC arm (27/29, 93.1%) was similar to that in the usual IV arm (26/28, 92.8%, p=0.958). Patient satisfaction at day 5 was better in the routine PICC arm (97%, ‘a little comfort’ or ‘much comfort’) compared with the usual IV arm (21%) (p < 0.001).
Oral administration of medication and nutrition is often difficult in terminally ill cancer patients because of progressive difficulties in swallowing, nausea and vomiting, intestinal obstruction, and consciousness disturbance [1]. Therefore, reliable intravenous (IV) access is an important issue in terminally ill cancer patients. However, these terminally ill cancer patients have limited or no peripheral venous access due to edema or repeated venous punctures from long-term IV therapy, including chemotherapy and blood transfusions. Thus, central venous access has provided an important role for IV access in terminally ill cancer patients.
There are several options for applying central venous catheters (CVCs) in cancer patients, including subclavian venous catheter, chemo-port (CP), and the peripherally inserted central catheter (PICC) approaches. Among these, PICC is well-tolerated insertion without catastrophic risk (e.g., pneumothorax or wound dehiscence) and provides medium-term intravascular access [2,3]. Terminally ill cancer patients are vulnerable to minor trauma due to poor performance and general conditions and may have behavior problems due to mental deterioration or delirium [4]. In addition, most of these patients have limited survival of 1–2 months [5,6]. Hence, terminally ill cancer patients need CVCs that are safe, comfortable to insert, and offer intermediate durability of IV access. Considering those aspects of terminally ill cancer patients and PICC, the PICC is an attractive alternative to other forms of CVCs.
However, limited data exist regarding the safety and efficacy of PICC in homogenous terminally ill cancer patients [7–9]. Our group previously conducted retrospective and prospective studies examining the performance of PICC in terminally ill cancer patients [7,8], demonstrating high insertion success rate (86%–100%), low premature removal rate (10%–16%), and favorable patient-reported satisfaction (80%). However, these previous studies had limitations to elucidate superiority of the PICC compared with peripheral IV access and appropriate time to insert PICC in terminally ill cancer patients due to the retrospective or single-arm design. Currently, when terminally ill cancer patients are admitted to the hospice-palliative care (HPC) unit, peripheral IV access is maintained as long as possible, and insertion of CVCs such as PICC are considered when it is no longer possible to access peripheral veins. However, many terminally ill cancer patients experience discomfort related to frequent venous puncture because of poor peripheral IV access at the time of admission. In addition, when PICC IV access is necessary, PICC insertion is often impossible due to poor medical condition such as coagulopathy or delirious behavioral problems. Thus, early insertion of PICC upon admission may be effective in patients requiring parenteral nutrition or medication.
The purpose of this study was to investigate whether routine insertion of PICC at the time of admission to a HPC unit is acceptable in terms of safety and efficacy and whether it results in superior patient satisfaction compared to usual IV access.
Terminally ill cancer patients who were admitted to the HPC unit at Pusan National University Yangsan Hospital between February 2017 and January 2020 were enrolled in this study. Terminally ill cancer patients receive no additional anti-cancer treatment in our institution and have estimated survival times of 1–2 months. Admission to the HPC unit is usually considered if parenteral nutrition and medication are required. Exclusion criteria were as follows: (1) patients who already had CVC such as CP, (2) patients with severe coagulopathies of platelet count less than 20,000 or internationalized normalized ratio higher than 2, (3) patients with evidence of overt sepsis, and (4) patients with severe behavioral problems that would make PICC insertion difficult. Patients with history of sepsis but negative culture test results and no signs of infection were allowed. If a prior PICC was removed due to unexpected events and reinserted, we did not count each PICC placement as a new event.
This study was a single-center, prospective, randomized phase II trial. The study subjects were admitted to the HPC unit between May 2017 and January 2020, stratified according to Eastern Cooperative Oncology Group (ECOG) performance status and previous infection history within 1 month, and randomly assigned to two groups: (1) the routine PICC arm (initial insertion of PICC at the time of admission, which means that the procedure was conducted within 3 working days after study enrollment), or (2) the usual IV access arm (maintenance of peripheral IV line until two trials a day, and late insertion of PICC). A research coordinator was responsible for randomizing patients to study arms using a computer-generated random allocation table with 4-block randomization.
All PICCs were inserted by an interventional radiologist in the angiography room using ultrasound guidance or fluoroscopic imaging. All operators wore aseptic gowns, masks, and gloves, and all of the patients received a dressing with aseptic drape. Seldinger’s technique was used routinely [10]. The PICC lines contained a single lumen and were made of second-/third-generation polyurethane. The location of the catheter tip was confirmed by chest radiography. None of the PICCs were sutured but were held in place with a StatLock Catheter Stabilization Device (BARD, Covington, GA).
No patient received prophylactic antibiotics or anticoagulation drugs for infection or thrombosis. Catheter replacement over a guidewire was strictly prohibited in this study. All patients received a closed dressing dampened with betadine on the catheter insertion site every 3 days. PICC tip cultures were performed when the catheter was removed at the time of discharge or within 30 minutes of death. If the catheter tip showed positive findings in culture, we checked for the presence of catheter-related blood stream infection (CRBSI) based on medical progress and laboratory findings.
We assessed clinical complications such as pain, edema, bleeding, and local or systemic catheter-related infections. CRBSIs were defined by positive catheter tip culture and at least one positive peripheral blood culture of the same organism without other sources of infection. Catheter-related thrombosis occurs in two types; intra-catheter thrombosis and thrombophlebitis. The former is suspected when the catheter flow rate slows or back flush is impossible, and the latter is suspected when patients complain of arm edema or pain.
In addition, we evaluated patient-perceived satisfaction using a semi-structured questionnaire assessing the degree of IV access related with comfort at day 5 of study enrollment: “How is your satisfaction with the IV access so far?” (rated as “much comfort,” “a little comfort,” “no change,” “a little discomfort,” or “much discomfort”). Patients who underwent PICC insertion were evaluated for procedure-related distress by the following question: “Did you experience distress because of the procedure?” (rated as “distressing,” “a little distressing,” or “not distressing”). Patients who underwent PICC in the usual IV arm were evaluated for comfort improvement at day 5 of PICC insertion by the following question: “How comfortable is the parenteral access after placement of the PICC?” (rated as “much more comfort,” “a little more comfort,” “no change,” “a little more discomfort,” or “much more discomfort”).
The sample size was calculated based on an assumption of maintenance success rate of routine PICC arm (90%) and usual IV arm (95%) based on prior study [7]. With a non-inferior margin of 25%, power of 80%, and alpha of 0.05, each of the two groups was determined to be 29 patients. Considering a drop-out rate of 10%, a total of 33 patients were planned to be randomized to each arm. Patients who died within 7 days of study enrollment or were transferred to other hospitals, those who required PICC insertion within 5 days of enrollment in the usual IV access arm, and those who did not receive PICC insertion within 3 days in the routine PICC arm were identified as drop-outs and excluded from the final analysis.
The baseline demographics and PICC-related characteristics of the patients were summarized using descriptive statistics, including median, mean, and range. The primary endpoint was IV maintenance success rate, defined as the ratio of patients who maintained functional IV access until the intended time (death or transfer) to all patients. In the routine PICC arm, IV maintenance success was defined when the PICC was maintained until the intended time. In the usual IV arm, IV maintenance success was defined when the peripheral IV line was maintained until the intended time without requiring PICC or PICC maintained until the intended time if inserted. If PICC was required in the usual IV arm but PICC insertion was impossible due to the patient’s general condition or coagulopathy, it was defined as IV maintenance failure. The secondary endpoints were the patients’ perceived satisfaction and complication rate. The complication rates were reported as complications per 1,000 PICC days and a simple rate. The IV maintenance success rate, which is the primary endpoint, was compared between the two groups using the Z-test. Kaplan-Meier estimates were used to analyze the time to event variable. Survival comparisons were performed using univariate log-rank tests. Median follow-up duration was calculated according to the inverted Kaplan-Meier method. Statistical analyses were performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL).
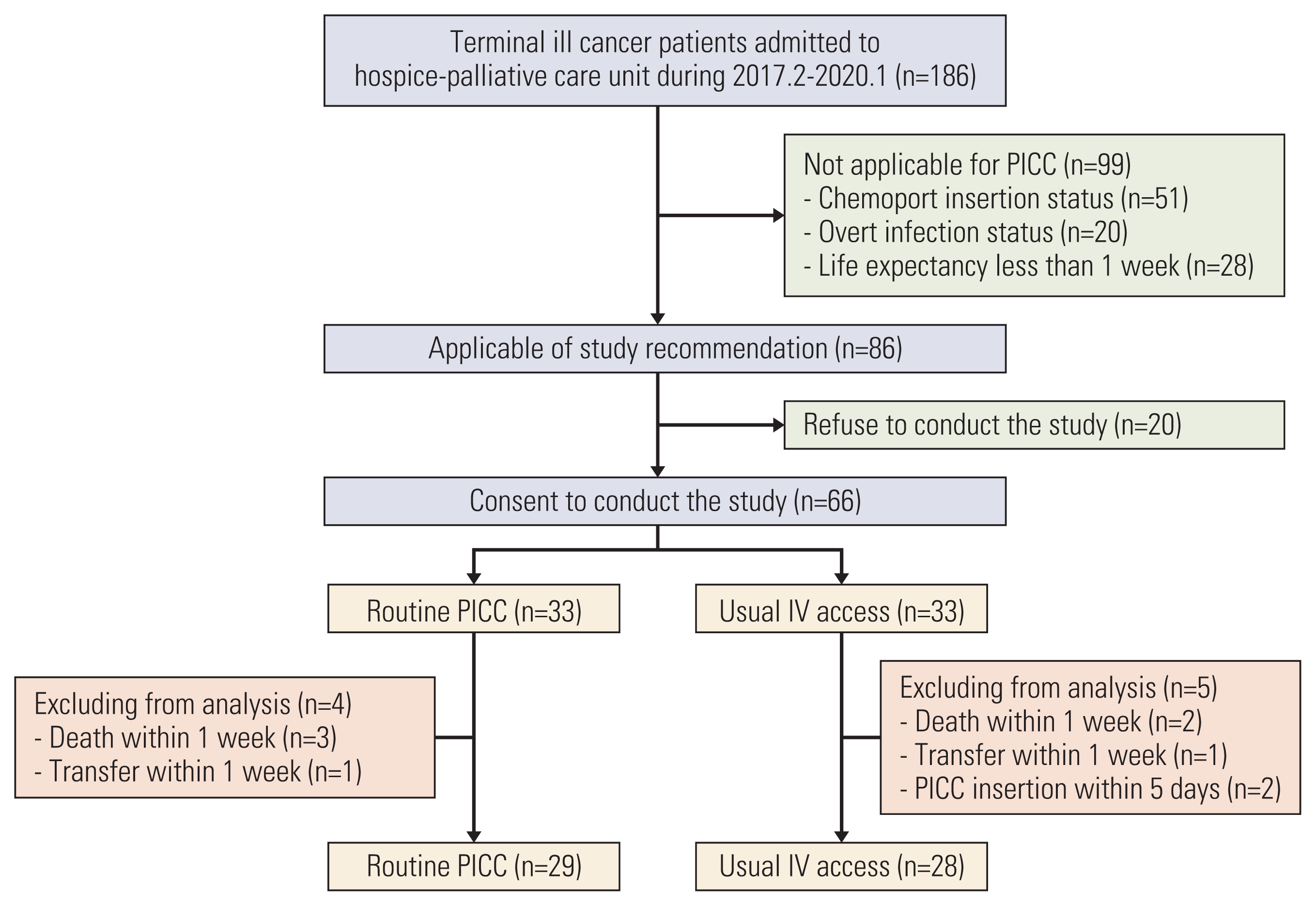
In total, 186 terminally ill cancer patients were admitted to the HPC unit during the study period, 66 patients of whom were enrolled in this study, and 33 patients in each group. In the routine PICC arm, 29 patients excluding those who died (3 patients) or were transferred (1 patient) within 1 week were analyzed. In the usual IV access arm, 28 patients were analyzed, excluding those who died (2 patients) or were transferred (1 patient) within 1 week, and two patients required PICC insertion within 5 days (Fig. 1). There was no difference in age, primary cancer site, or prior curative treatment between the two groups. There were no differences in factors indicating general medical condition and life expectancy such as ECOG performance status and simplified palliative prognostic index. Prior infection and previous central venous access performance were similar between the two groups (Table 1).
In the routine PICC arm, 27 of the 29 cases maintained PICC until the intended time (death, 23; transfer, 4); and the other two patients prematurely removed PICC due to delirium-related self-removal. In the usual IV arm, 11 patients maintained peripheral IV access until death, 15 patients underwent PICC insertion and maintained it until the intended time (death 13, transfer 2), and the other two patients were unable to undergo PICC due to poor medical condition. In summary, the IV maintenance success rate in the routine PICC arm (27/29, 93.1%) was similar to that in the usual IV arm (26/28, 92.8%, p=0.958) (Table 2).
In the 44 PICC insertion cases, the mean time from admission to the HPC unit to PICC insertion was 0.84 days (range, 0 to 3 days) in the routine PICC arm and 8.65 days (range, 6 to 18 days) in the usual IV arm. PICCs were successfully inserted in all patients without immediate catastrophic complications. Procedure-related distress was mostly “not distressing” (76% in routine PICC group, 87% in usual IV group) or “a little distressing” (17% vs. 13%) in both arms. The median catheter life span was 16.0 days (95% confidence interval [CI], 8.5 to 23.5) in the routine PICC arm and 8.0 days (95% CI, 4.5 to 11.5) in the usual IV arm (Table 3). With median follow-up duration of 55.0 days (95% CI, 31.1 to 78.8), the median overall survival from admission to death was 16.0 days (95% CI, 10.7 to 21.3) in the routine PICC arm and 15.6 days (95% CI, 13.5 to 16.5) in the usual IV access arm (Fig. 2).
Regarding satisfaction with IV access on day 5 of study enrollment, patient-reported comfort levels were as follows in the routine PICC arm: much comfort (n=14, 48%) and a little comfort (n=14, 48%), with most patients (96%) reporting favorable satisfaction. In the usual IV arm, only 7% and 14% of patients reported much comfort and a little comfort, respectively, while 50% of patients reported no change and 25% reported a little discomfort. The routine PICC access group reported significantly better comport compared to the usual IV access group (p < 0.001). Among the 15 patients who actually underwent PICC in the usual IV arm, all answered that they were more comfortable with IV access after PICC insertion (Table 4).
Nine complications (28%, 14.1/1,000 PICC days) occurred in the routine PICC arm, while six complications (40%, 33.9/1,000 PICC days) occurred in the usual IV access arm. The most frequently documented complication was bleeding in nine cases, followed by feeling of irritation and self-removal in two cases. Cases with bleeding were only trivial bleeding and were resolved by simple compression (Table 5). The mean time from PICC insertion to complication occurrence was 21.2 days (range, 3 to 57 days), except for bleeding complications which mostly occurred immediately after PICC insertion (mean, 2.2 days; range 1 to 8 days). There was no PICC complication-related death. Among the 36 cases (routine PICC arm, 23 cases; usual IV access arm, 15 cases) in whom PICCs remained positioned until death, the catheter tip was cultured in 18 cases and 10 cases, respectively. One case (6%) in the routine PICC arm had positive tip culture results, and the pathogen was Staphylococcus aureus. The patient was started on palliative sedation with intractable dyspnea due to cancer progression on day 52 of PICC insertion, and fever occurred on day 57. The clinicians focused on symptom control rather than the additional work-up considering systemic conditions, and the patient died on day 60. We concluded a diagnosis of CRBSI based on clinical data, although the patient did not fulfill definitive criteria.
The current study showed effectiveness and safety of routine PICC insertion at the time of hospitalization for HPC, because routine initial PICC insertion does not increase complications compared to the usual IV access (i.e., delayed PICC) and has a similar IV maintenance success rate. Additionally, patient-perceived satisfactions in the routine PICC arm was significantly more favorable than that in the usual IV access arm. Thus, this study showed that PICC could be routinely inserted at admission to the HPC unit in terminally ill cancer patients, considering their poor general conditions and limited period of survival.
Even though PICC insertion was performed routinely at the time of hospitalization, it was maintained at 90% or higher until the intended time, and these values were not different from those of the usual IV access arm. This is in line with the good PICC performance observed in terminally ill cancer patients in our previous study [7]. On the other hand, one case of CRBSI (day 57) and two cases of self-removal (days 8 and 34), which can lead to premature removal, occurred only in the routine PICC group. This suggests increased risk of routine PICC insertion-related complications according to longer dwelling time of PICC, based on previous studies showing that increases in the maintaining period of PICC were correlated with higher incidence of complications [9]. However, even in the routine PICC group, the probability of CRBSI or premature removal was very low compared to those in previous studies investigating PICC [9,11,12]. Another important finding was that the routine PICC group showed significant patient’s perceived satisfaction with respect to IV access compared to the usual IV access. This is an expected result considering that peripheral IV access is often difficult in HPC patients who are elderly or have undergone repeated puncture for long periods of time. Considering the excellent maintenance success rate of PICC, patient satisfaction with routine PICC, and the comprehensive goal of HPC being focus on patient quality of life, the IV access strategy using routine PICC at the admission of HPC unit could be effective.
As in our previous studies [7], the current study showed a PICC success rate of 100%, and there were no serious procedure-related complications during PICC insertion. Additionally, PICC insertion-related distress was trivial in both study arms. These favorable results may be due to the performance of all procedures by experienced interventional radiologists using radiologic guidance; therefore, a superior success rate and safety [12–14]. The success rate and safety at the time of PICC insertion are important in terminally ill cancer patients because they are in poor general status and vulnerable to trivial damage. Our results strongly support the benefits of early PICC insertion.
In this study, there were significantly fewer PICC-related complications than in previous studies [9,11–14]. The reasons are presumed to the following characteristics of the current study; PICC insertion was performed by an experienced radiologist using ultrasound guidance or fluoroscopic imaging under strict sterile conditions rather than by bed-side blind insertion, patients with hematologic malignancies associated with relatively high risk of adverse events were excluded, and strict PICC management and monitoring related to the characteristics of the prospective trial. Above all, considering that the mean time to occurrence of complications was 21.2 days, the limited life span of terminally ill cancer patients in the HPC (mean survival, 22.2 days) would be a major explanation of lack of complication. Therefore, our results suggest that routine PICC should be applied under strict PICC management can be performed in terminally ill cancer patients with limited lifespans.
The major limitation of this study was possibility of bias due to its nature as a clinical audit study and relatively small sample size single-center study. First, the measurement tool for assessing patient satisfaction by a study coordinator may result in an underestimation of patient-reported distress and overestimation of patient-reported usefulness. Nevertheless, we tried to minimize physician bias by including outcome evaluations conducted by another team’s independent rater. Second, the sample size may not be sufficient to represent actual complication rates. Moreover, tip cultures were not obtained for all cases, and detailed evaluations were limited due to the poor general performance of terminally ill cancer patients. Therefore, considering the relatively small sample size, single center, limited survival duration, and procedure performed by interventional radiologists, the current study results need cautious generalization for overall HPC patients. The routine PICC could be considered in terminally ill cancer patients with limited lifespans under hospital settings where PICC insertion and management can be appropriately performed.
Despite these limitations, this study was the first randomized phase II study of PICC in terminally ill cancer patients using an active comparator. The study showed that more than 90% of routine PICC-inserted patients maintained the PICC with function until the intended time, and 97% reported satisfaction with IV access compared to 21% of satisfaction with usual IV access. Considering the characteristics of terminally ill cancer patients, such as poor general condition and limited period of survival, initial PICC insertion at the time of admission to the HPC is a safe and useful option for IV access.
In conclusion, routine PICC insertion in terminally ill cancer patients showed comparable safety and efficacy and superior satisfaction compared with usual IV access. Thus, routine PICC insertion could be considered at admission to the HPC unit.
Notes
Ethical Statement
Written informed consent was obtained from all patients or their proxies. This study was approved by the institutional review board (05-2016-148) and registered at ClinicalTrials.gov (NCT03299868).
Author Contributions
Conceived and designed the analysis: Park K.
Collected the data: Park EJ, Park K, Kim JJ, Oh SB, Jung KS, Oh SY, Hong YJ, Kim JH, Jang JY, Jeon UB.
Contributed data or analysis tools: Park EJ, Park K, Kim JJ, Jung KS.
Performed the analysis: Park K, Oh SB, Oh SY.
Wrote the paper: Park EJ, Park K, Hong YJ.
PICC procedure: Kim JH, Jang JY, Jeon UB.
Acknowledgments
This study was supported by Research Institute for Convergence of biomedical science and technology Grant (30-2017-004), Pusan National University Yangsan Hospital.
References
2. Walshe LJ, Malak SF, Eagan J, Sepkowitz KA. Complication rates among cancer patients with peripherally inserted central catheters. J Clin Oncol. 2002; 20:3276–81.

3. Ng PK, Ault MJ, Ellrodt AG, Maldonado L. Peripherally inserted central catheters in general medicine. Mayo Clin Proc. 1997; 72:225–33.

4. Lundh Hagelin C, Seiger A, Furst CJ. Quality of life in terminal care: with special reference to age, gender and marital status. Support Care Cancer. 2006; 14:320–8.
5. Lee GJ, Ahn HS, Go SE, Kim JH, Seo MW, Kang SH, et al. Patient’s factors at entering hospice affecting length of survival in a hospice center. Cancer Res Treat. 2015; 47:1–8.

6. Llobera J, Esteva M, Rifa J, Benito E, Terrasa J, Rojas C, et al. Terminal cancer. duration and prediction of survival time. Eur J Cancer. 2000; 36:2036–43.
7. Park K, Jun HJ, Oh SY. Safety, efficacy, and patient-perceived satisfaction of peripherally inserted central catheters in terminally ill cancer patients: a prospective multicenter observational study. Support Care Cancer. 2016; 24:4987–92.

8. Park K, Lim HG, Hong JY, Song H. Safety and efficacy of peripherally inserted central catheters in terminally Ill cancer patients: single institute experience. Korean J Hosp Palliat Care. 2014; 17:179–84.

9. Yamada R, Morita T, Yashiro E, Otani H, Amano K, Tei Y, et al. Patient-reported usefulness of peripherally inserted central venous catheters in terminally ill cancer patients. J Pain Symptom Manage. 2010; 40:60–6.

10. McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003; 348:1123–33.

11. Gunst M, Matsushima K, Vanek S, Gunst R, Shafi S, Frankel H. Peripherally inserted central catheters may lower the incidence of catheter-related blood stream infections in patients in surgical intensive care units. Surg Infect (Larchmt). 2011; 12:279–82.

12. Cheong K, Perry D, Karapetis C, Koczwara B. High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours. Intern Med J. 2004; 34:234–8.

Fig. 2
Overall survival according to intravenous access. IV, intravenous; PICC, peripherally inserted central catheter.
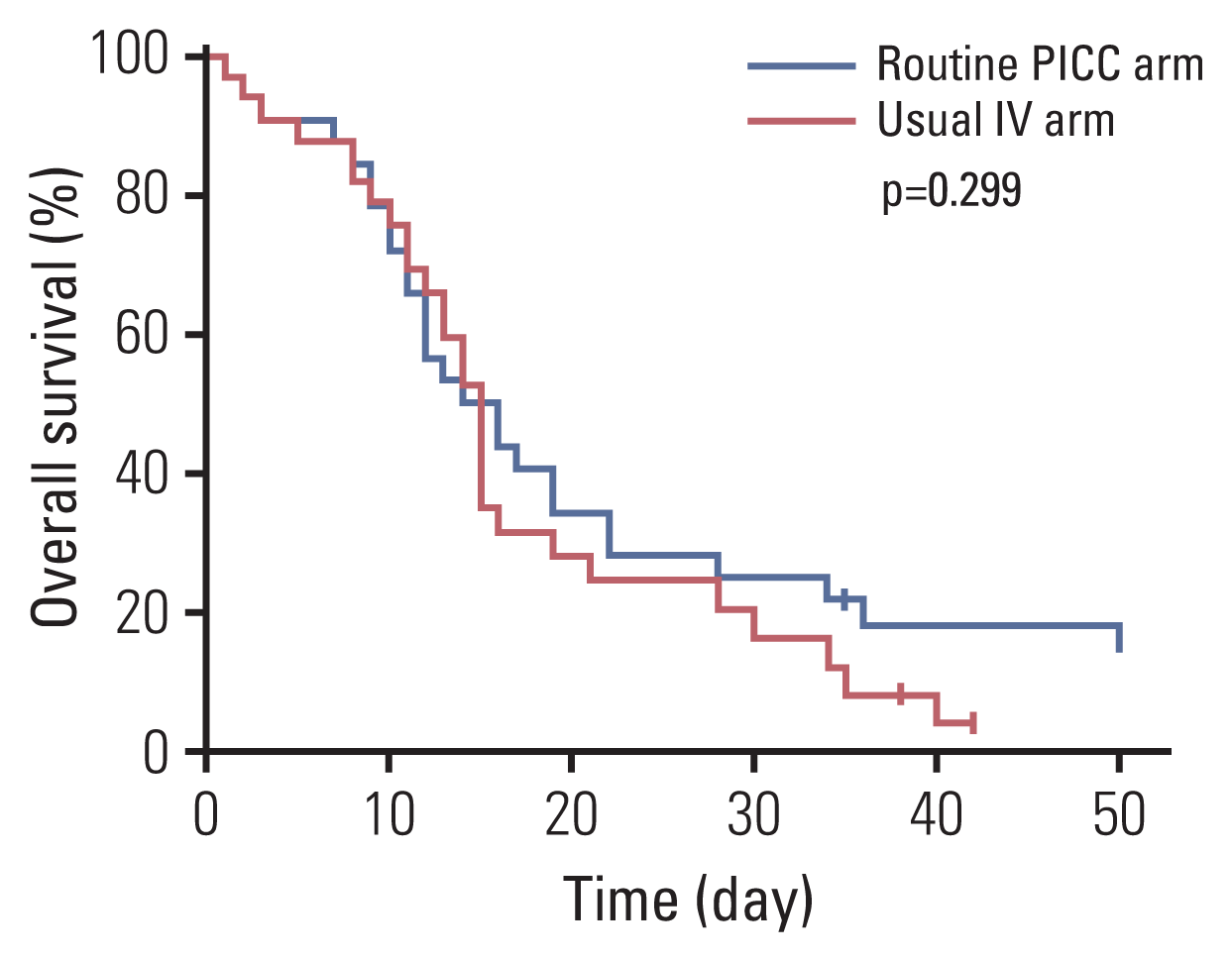
Table 1
Baseline characteristics
| Characteristic | Routine PICC (n=29) | Usual IV access (n=28) | p-value |
|---|---|---|---|
| Age, mean (range, yr) | 66.2 (49–85) | 70.1 (58–87) | 0.058 |
| Sex | |||
| Male | 24 (83) | 21 (75) | 0.530 |
| Female | 5 (17) | 7 (25) | |
| Primary cancer type | |||
| Gastrointestinal | 1 (3) | 3 (11) | 0.738 |
| Hepatobiliary, pancreas | 17 (59) | 12 (43) | |
| Lung | 2 (7) | 2 (7) | |
| Genitourinary | 6 (21) | 8 (29) | |
| Sarcoma | 3 (10) | 2 (7) | |
| Melanoma | 0 | 1 (4) | |
| Prior curative treatment | |||
| Initially metastatic | 23 (79) | 19 (68) | 0.379 |
| Recurrent | 6 (21) | 9 (32) | |
| ECOG performance scale | |||
| 2 | 6 (21) | 4 (14) | 0.874 |
| 3 | 16 (55) | 17 (61) | |
| 4 | 7 (24) | 7 (25) | |
| sPPI, mean (95% CI) | 7.0 (5.8–8.1) | 7.6 (6.2–9.1) | 0.505 |
| Infectious status | |||
| None | 26 (90) | 25 (89) | > 0.99 |
| Previous bacteremiaa) | 3 (10) | 3 (11) | |
| Previous central venous access | |||
| None | 24 (83) | 22 (79) | 0.458 |
| PICC | 1 (3) | 1 (4) | |
| Chemo-port | 1 (3) | 4 (14) | |
| CVC (jugular or subclavian) | 3 (10) | 1 (4) | |
Table 2
Results of IV access
| Characteristic | Routine PICC (n=29) | Usual IV access (n=28) | p-value |
|---|---|---|---|
| Peripheral IV maintenance | NA | 11 | |
| PICC insertion faileda) | 0 | 2 | |
| Causes of PICC removalb) | 29 | 15 | |
| Deaths | 23 | 13 | |
| Transfer to another hospital | 4 | 2 | |
| Self-removal | 2 | 0 | |
| IV maintenance success rate (%) | 93.1 | 92.8 | 0.958 |
Table 3
Results of PICC (n=44a))
| Characteristic | Routine PICC (n=29) | Usual IV access (n=15) | p-value |
|---|---|---|---|
| Time to PICC insertion (day) | 0.84 (0–3) | 8.65 (6–18) | < 0.001 |
| Complication at the time of insertion | |||
| None | 21 (72) | 12 (80) | 0.722 |
| Bleeding | 8 (28) | 3 (20) | |
| Arterial puncture | 0 | 0 | |
| Hemothorax/Pneumothorax | 0( | 0 | |
| Utilization of PICCb) | |||
| TPN | 27 (93) | 14 (93) | > 0.99 |
| Simple hydration | 23 (79) | 14 (93) | |
| Parenteral administration of medication | 29 (100) | 15 (100) | |
| Blood product transfusion | 5 (17) | 2 (13) | |
| Procedure-related distress | |||
| None distress | 22 (76) | 13 (87) | 0.848 |
| A little distress | 5 (17) | 2 (13) | |
| Distress | 2 (7) | 0 | |
| Results of PICC removal | |||
| Deaths | 24 (83) | 13 (87) | 0.839 |
| Self-removal | 2 (7) | 0 | |
| Discharge | 0 | 0 | |
| Transfer to another hospital | 3 (10) | 2 (13) | |
| PICC life span (day) | 16.0 (7–60) | 8.0 (1–34) | 0.038 |
Table 4
Patient-reported satisfaction about intravenous access
| Characteristic | Routine PICC (n=29) | Usual IV access (n=28) | p-value |
|---|---|---|---|
| Satisfaction of intravenous access at day 5 of study enroll | < 0.001 | ||
| Much comfort | 14 (48) | 2 (7) | |
| A little comfort | 14 (48) | 3 (14) | |
| Neutral | 1 (3) | 15 (50) | |
| A little discomfort | 0 | 7 (25) | |
| Much discomfort | 0 | 0( | |
| NA | 0 | 1 (4)a) | |
| Satisfaction of IV access at day 5 after PICC insertb) | |||
| Neutral → Much comfort | - | 6 (40) | |
| Neutral → A little comfort | - | 5 (34) | |
| A little discomfort → Much comfort | - | 2 (13) | |
| A little discomfort → A little comfort | - | 2 (13) |
Table 5
PICC-related adverse events
| Routine PICC arm (n=29) | Usual IV access arm (n=15) | |||
|---|---|---|---|---|
|
|
|
|||
| No. (%) | Rate (per 1,000 PICC days) | No. (%) | Rate (per 1,000 PICC days) | |
| No complication | 21 (72) | 10 (67) | ||
|
|
||||
| Complicationa) | ||||
|
|
||||
| CRBSI | 1 (3) | 1.6 | 0 | 0 |
|
|
||||
| Intra-catheter thrombosis | 0 | 0 | 0 | 0 |
|
|
||||
| Thrombophlebitis | 0 | 0 | 0 | 0 |
|
|
||||
| Bleeding | 5 (17) | 7.8 | 4 (27) | 22.6 |
|
|
||||
| Feeling of irritation | 1 (3) | 1.6 | 2 (13) | 11.3 |
|
|
||||
| Self-removal | 2 (7) | 3.1 | 0 | 0 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download