Abstract
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: MKRS, MKLP, MAK, AH, NH, MKC, SP. Data curation: MKRS, MKLP, MAK, AH, NH, MKC, SP, MSJ, SL, VS, JK, BE, EB. Formal analysis: MKRS, MKLP, MAK, AH, NH, MKC, SP. Methodology: MAK, MKRS, AH, NH, MKC, SP. Project administration: MKRS, MKLP, MSJ, OJI. Visualization: MKRS, MSJ, SL, VS, JK, BE, EB. Writing–original draft: MKRS, MKLP, MAK, AH, NH, MKC, SP, MSJ, SL, VS, JK, BE, EB. Writing–review & editing: all authors.
SUPPLEMENTARY MATERIALS
Supplementary Figure 1.
Supplementary Figure 2.
REFERENCES
Figure 2.
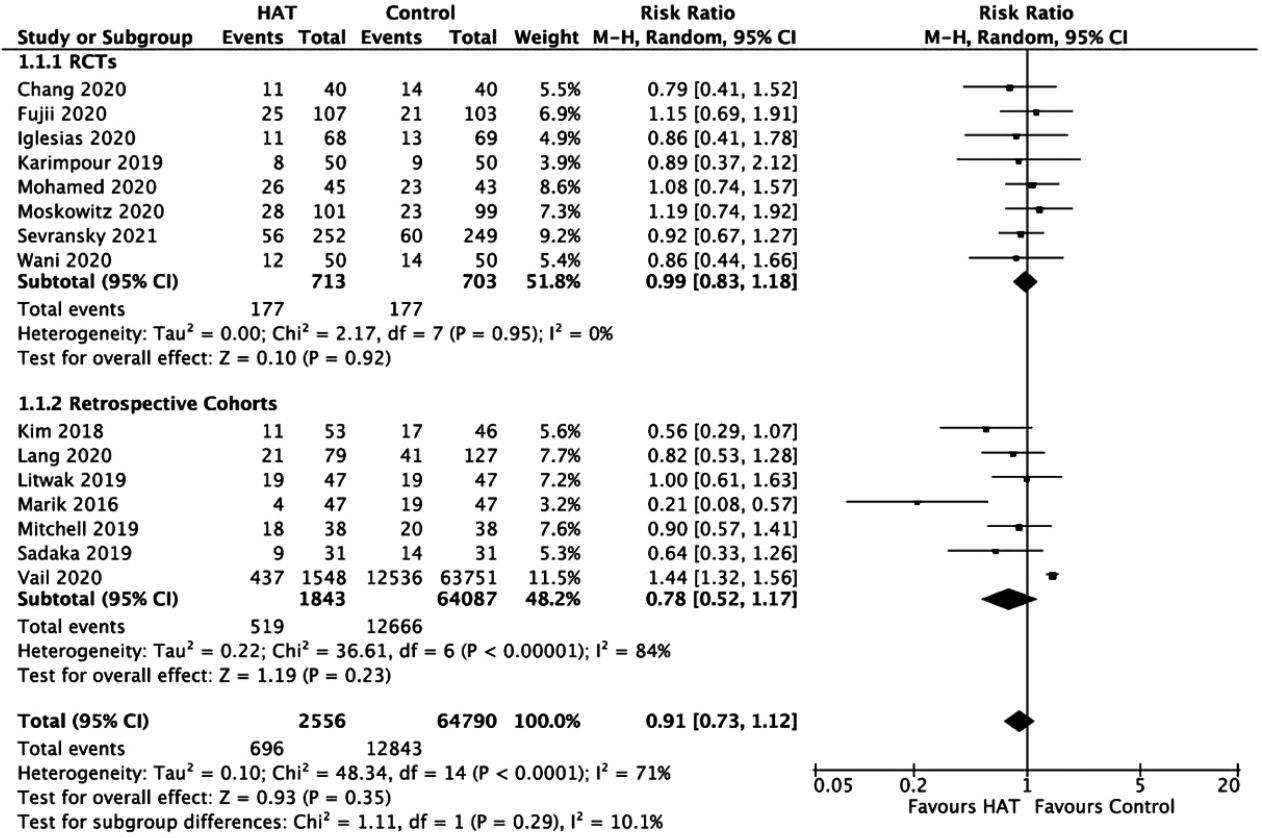
Figure 3.
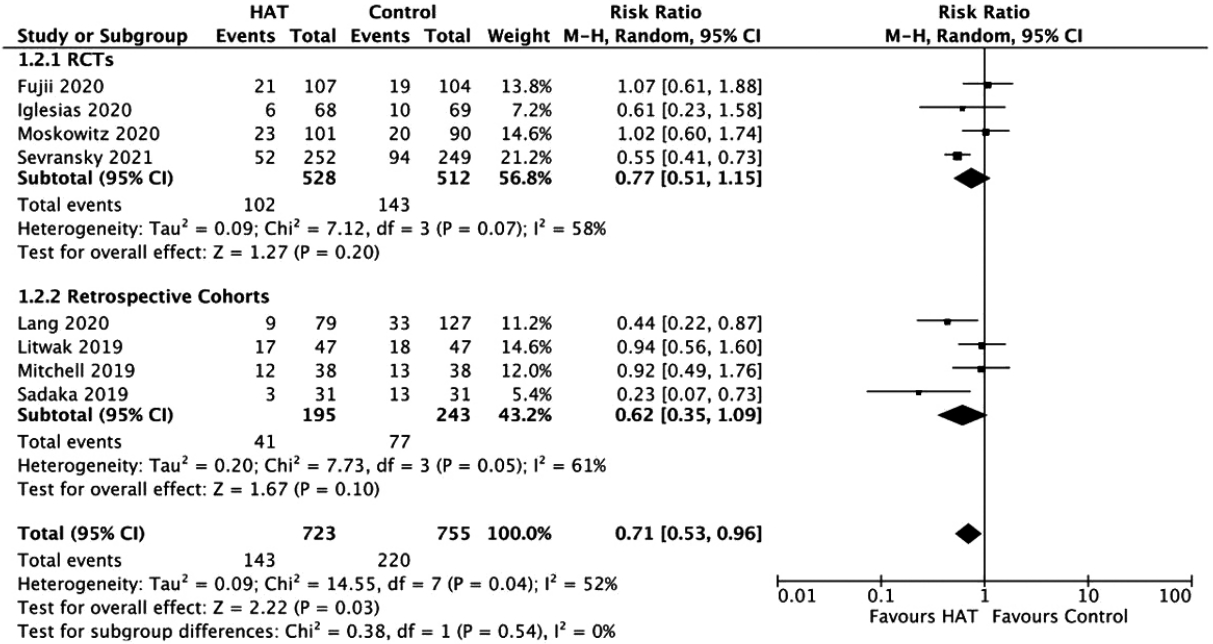
Figure 4.
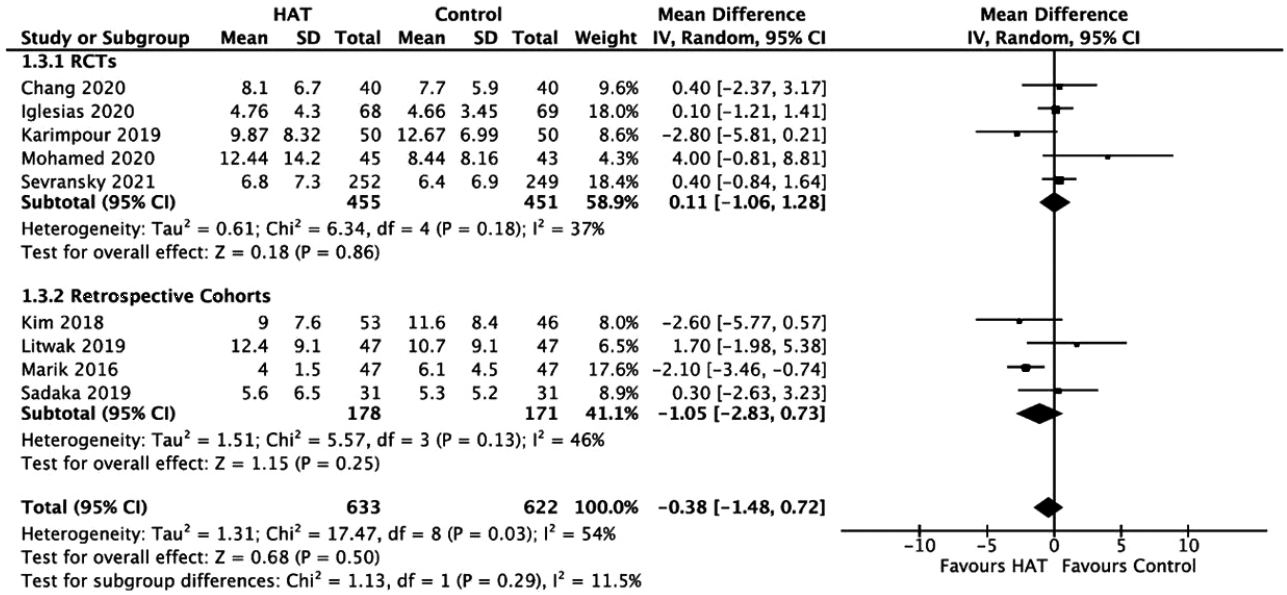
Figure 5.
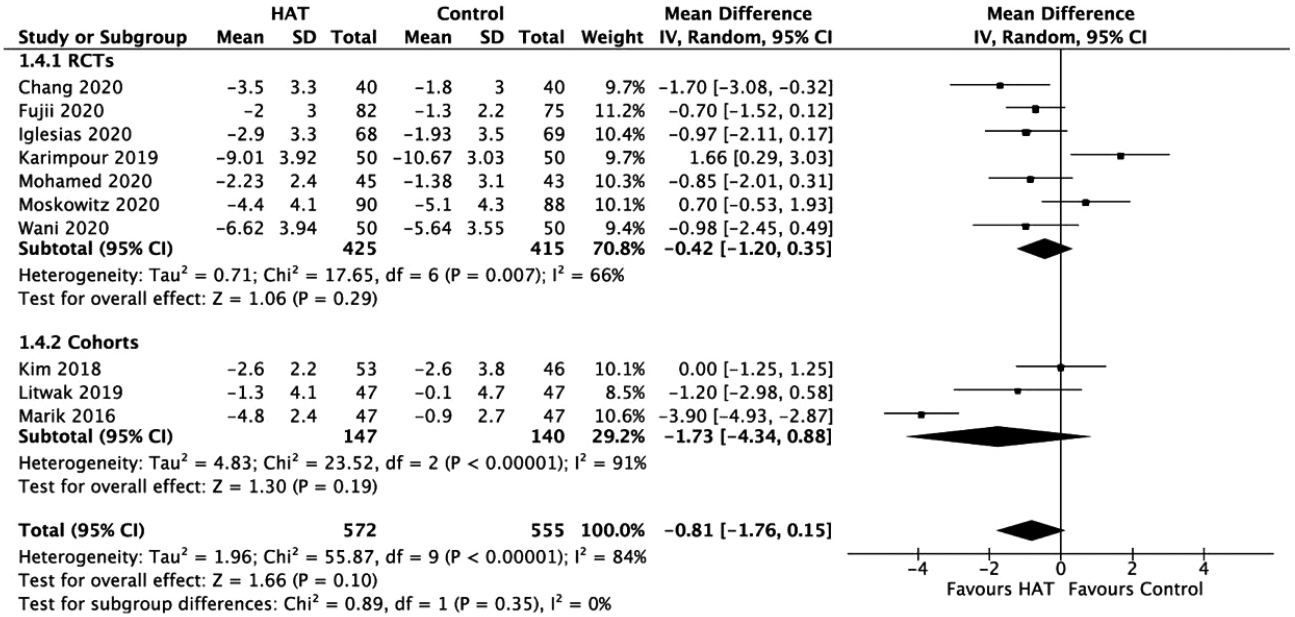
Figure 6.
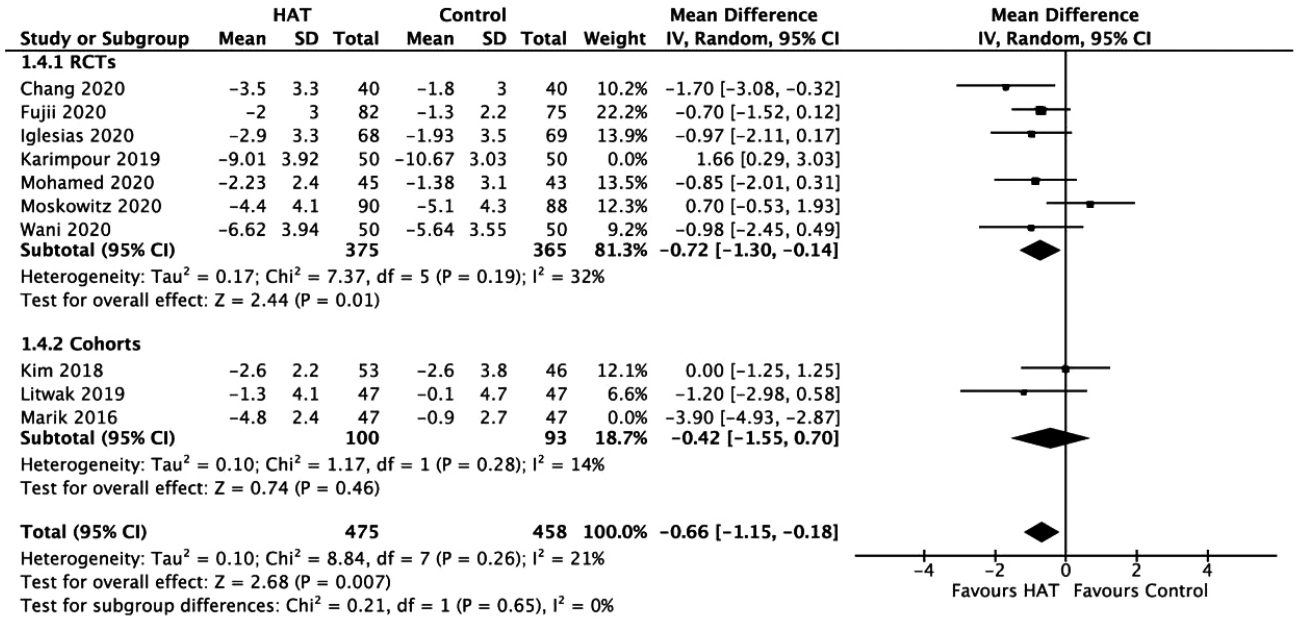
Figure 7.
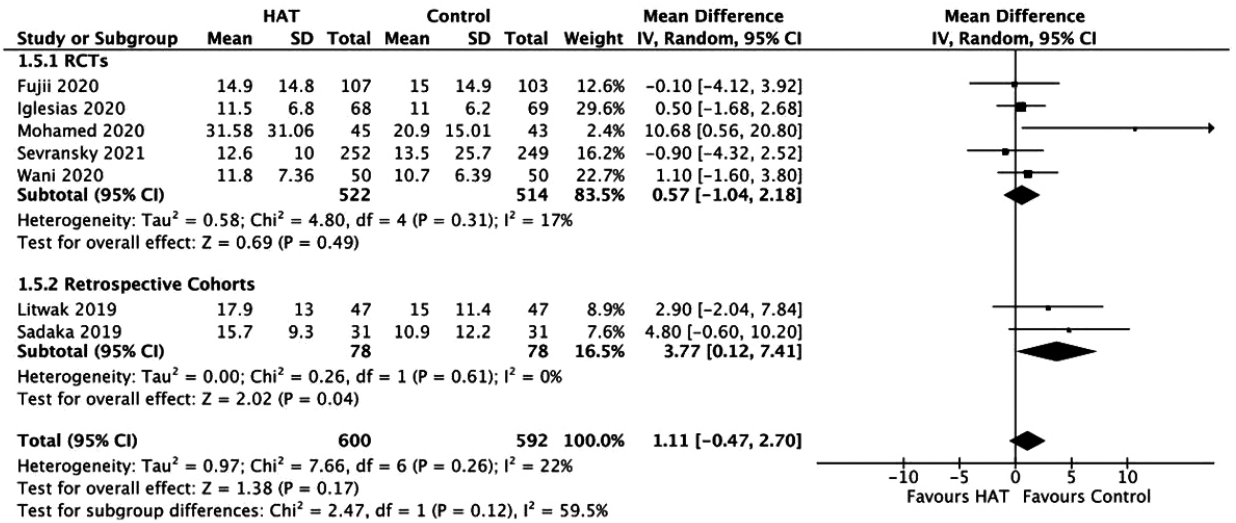
Figure 8.
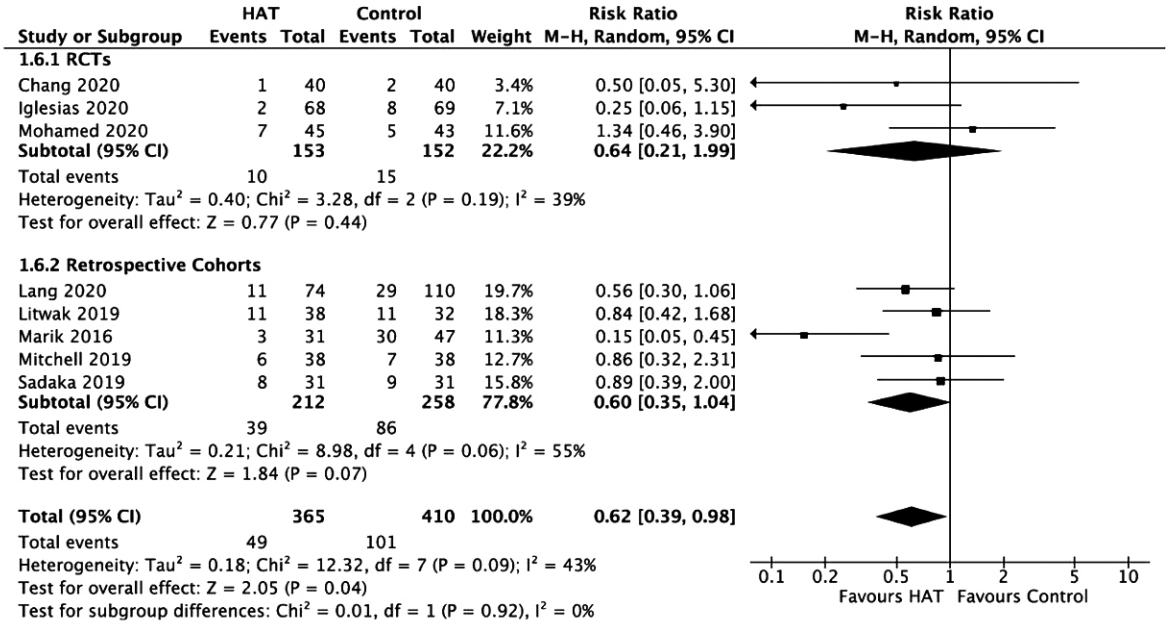
Figure 9.
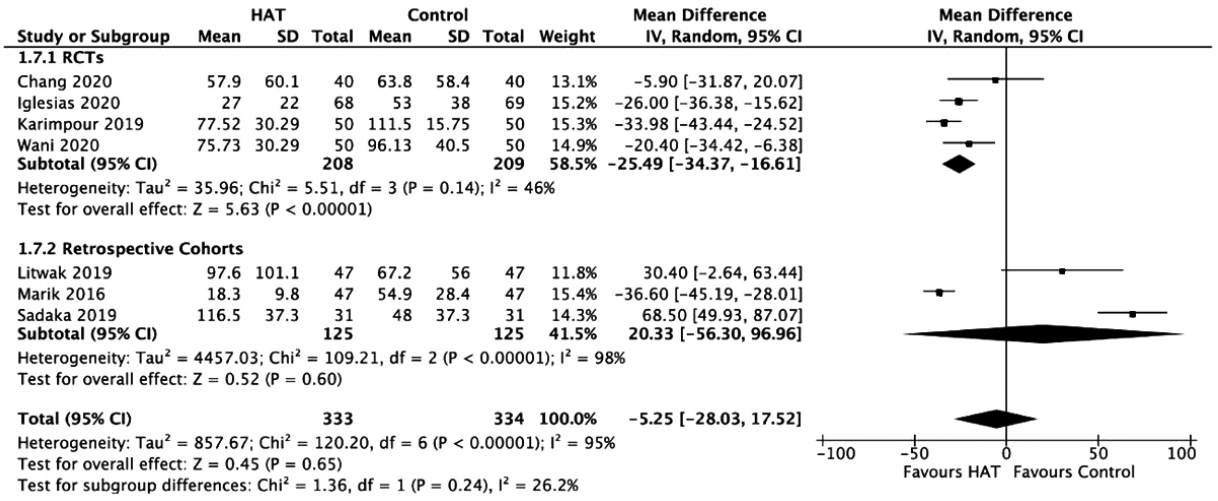
Table 1.
| Study | Country | Study design | Total sample |
Sample |
Age (yr) |
Male |
Control group treatment | Intervention and dosage/route | Outcome in the intervention group | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | Intervention group | Control group | Intervention group | Control group | |||||||
| Sadaka (2019) [38] | USA | Retrospective | 62 | 31 | 31 | 67±16 | 70±12 | 16 (52) | 16 (52) | Managed according to current sepsis guidelines | Ascorbic acid 1.5 g IV every 6 hours for 4 days; hydrocortisone 50 mg IV every 6 hours for 7 days; thiamine 200 mg IV every 12 hours for 4 days IV | ICU mortality, 3 (9.6) |
| Hospital mortality, 9 (29) | ||||||||||||
| Hospital LOS, 15 days (10–22) | ||||||||||||
| RRT for AKI, 8 (26) | ||||||||||||
| Duration of VP, 4.5 days (4–6) | ||||||||||||
| MV-free day, 10.2 (5–15) | ||||||||||||
| Marik (2016) [39] | USA | Retrospective | 94 | 27 | 47 | 58.3±14.1 | 62.2±14.3 | 27 (100) | 23 (49) | Broad spectrum antibiotics, fluids, vasopressors, enteral nutrition, thrombosis prophylaxis, allow permissive hyperglycemia | Ascorbic acid 1.5 g IV every 6 hours for 4 days or until ICU discharge; hydrocortisone 50 mg IV every 6 hours for 7 days or until ICU discharge; thiamine 200 mg IV every 12 hours for 4 days or until ICU discharge | Hospital mortality, 4 (8.5) |
| ICU LOS, 4 days (3–5) | ||||||||||||
| Duration of VP, 18.3±9.8 hours | ||||||||||||
| RRT for AKI, 3/31 (10) | ||||||||||||
| Procalcitonin clearance (72 hours), 86.4% (80.1%–90.8%) | ||||||||||||
| Mitchell (2019) [40] | USA | Retrospective | 76 | 38 | 38 | 68±10 | 68±10 | 36 (95) | 37 (97) | IV hydrocortisone alone | Ascorbic acid 1.5 g IV every 6 hours for 4 days; hydrocortisone 50 mg IV every 6 hours or 100 mg IV every 8 hours or continuous IV infusion of 10 mg per hour for 7 days then tapered over approximately 3–5 days; thiamine 200 mg IV every 12 hours for 4 days | Hospital mortality, 18 (47) |
| ICU mortality, 12 (32) | ||||||||||||
| 28-Day mortality, 22 (58) | ||||||||||||
| 60-Day mortality, 22 (58) | ||||||||||||
| Litwak (2019) [41] | USA | Retrospective | 94 | 47 | 47 | 58.3±17 | 60.1±14 | 28 (59.6) | 29 (61.7) | Fluid resuscitation with 30 mL/kg of crystalloid, broad spectrum antibiotics, vasopressor therapy | At least one dosage of each of the following medications; ascorbic acid 1.5 g IV every 6 hours; hydrocortisone 50 mg IV every 6 hours or 100 mg IV every 8 hours; thiamine 200 mg IV every 12 hours | Hospital mortality, 19 (40.4) |
| ICU mortality, 17 (36.2) | ||||||||||||
| RRT for AKI, 11/38 (28.9) | ||||||||||||
| ICU LOS, 11.0 days (7.0–19.0) | ||||||||||||
| Hospital LOS, 19.0 days (9.0–26.) | ||||||||||||
| Duration of VP, 84.2 hours (37.0–169.3) | ||||||||||||
| Delta procalcitonin (72 hours), 0.1 ng/mL (–55.0 to 9.1) | ||||||||||||
| Fujii (2020) [42] | Australia, New Zealand and Brazil | RCT | 211a | 107 | 104 | 61.9±15.9 | 61.6±13.9 | 68 (63.6) | 65 (62.5) | IV hydrocortisone 50 mg every 6 hours and thiamine 200 mg every 12 hours | Ascorbic acid 1.5 g IV every 6 hours; hydrocortisone 50 mg IV every 6 hours; thiamine 200 mg every 12 hours | 28-Day mortality, 24/106 (22.6) |
| 90-Day mortality, 30/105 (28.6) | ||||||||||||
| ICU mortality, 21 (19.6) | ||||||||||||
| Hospital mortality, 25 (23.4) | ||||||||||||
| 28-Day cumulative vasopressorfree days, 25.6 (17.8–26.8) | ||||||||||||
| 28-Day cumulative mechanical ventilation-free days, 25.3 (5.2–28.0) | ||||||||||||
| 28-Day RRT–free days, 28.0 (23.5–28.0) | ||||||||||||
| 28-Day ICU-free days, 21.9 (0–25.8) | ||||||||||||
| Hospital LOS, 12.3 days (6.2–26.0) | ||||||||||||
| Karimpour (2019) [43] | Iran | RCT | 100 | 50 | 50 | 56.2±13.6 | 61.1±16.9 | 23 (46) | 20 (40) | Normal saline, norepinephrine, hydrocortisone | Ascorbic acid 50 mg/kg IV every 6 hours up to 6 g/day for 4 days; all included patients received hydrocortisone 200 mg daily for 4 days then tapers over 4 days; thiamine 200 mg IV | Delta SOFA score, 9.01±3.92 |
| 28-Day mortality, 8 (15) | ||||||||||||
| Duration of vasopressors, 77.52±21.5 hours | ||||||||||||
| ICU LOS, 9.87±8.32 | ||||||||||||
| Mechanical ventilation, 6.67±7.84 days | ||||||||||||
| Procalcitonin level, 1.25±1.61 ng/mL | ||||||||||||
| Wani (2020) [44] | India | RCT | 100 | 50 | 50 | 51.5±35.6 | 50.7±35.5 | 31 (62) | 28 (56) | Broad spectrum antibiotics, intravenous fluids, vasopressors, and mechanical ventilation as indicated | Ascorbic acid 1.5 g IV every 6 hours for 4 days or until hospital discharge; hydrocortisone 50 mg IV every 6 hours for 7 days or until ICU discharge then tapered over 3 days; thiamine 200 mg IV every 12 hours for 4 days or until hospital discharge | Hospital mortality, 24% |
| Duration of vasopressor use, 75.72±30.29 | ||||||||||||
| Hospital LOS, 11.82±7.36 days | ||||||||||||
| 30-Day mortality, 20 (40) | ||||||||||||
| Kim (2018) [45] | Korea | Retrospective | 99 | 53 | 46 | 73 (62–79) | 74 (68–79) | 41 (77) | 29 (63) | Managed according to therapeutic recommendations in Surviving Sepsis Campaign Guidelines and lungprotective ventilation strategy | Ascorbic acid 6 g IV divided into 4 equal doses; hydrocortisone 50 mg IV every 6 hours for 7 days then tapered over 3 days; thiamine 200 mg IV every 12 hours for 4 days | Hospital mortality, 6 (17) |
| ICU LOS, 9 days (5–14) | ||||||||||||
| No. ventilator-free days at day 28, 12.3±11.0 | ||||||||||||
| No. vasopressor-free days at day 28, 19.8±10.8 | ||||||||||||
| Iglesias (2020) [46] | USA | RCT | 137 | 68 | 69 | 70±12 | 67±14 | 32 (47) | 27 (39) | Saline placebo | Ascorbic acid 1.5 g every 6 hours; hydrocortisone 50 mg every 6 hours; thiamine 200 mg every 12 hours | Delta SOFA score at 72 hours, 2.9±3.3 |
| Duration of vasopressors, 27±22 hours | ||||||||||||
| Hospital mortality, 11 (16) | ||||||||||||
| ICU mortality, 6 (9) | ||||||||||||
| Hospital LOS, 11.5±6.8 days | ||||||||||||
| ICU LOS, 4.76±4.3 days | ||||||||||||
| Procalcitonin clearance, 63 (170) | ||||||||||||
| Ventilator-free days, 22 (6.2) | ||||||||||||
| AKI, 54 (79) | ||||||||||||
| Moskowitz (2020) [47] | USA | RCT | 200 | 101 | 99 | 68.9±15 | 67.6±13.9 | 57 (56.4) | 54 (54.6) | Local sepsis guidelines including antibiotics, volume resuscitation and vasopressors | Ascorbic acid 1.5 g every 6 hours for 4 days or until ICU discharge; hydrocortisone 50 mg every 6 hours for 4 days or until ICU discharge; thiamine 100 mg every 6 hours for 4 days or until ICU discharge | All-cause mortality over 30 days, 35 (34.7) |
| Kidney failure, 32 (31.7) | ||||||||||||
| Ventilator-free days, 6 (2–7) | ||||||||||||
| Shock-free days, 5 (3–5) | ||||||||||||
| Incidence of delirium, 31/83 (37.4) | ||||||||||||
| ICU-free days, 22 (3–25) | ||||||||||||
| All-cause mortality to ICU discharge, 23 (22.7) | ||||||||||||
| All-cause mortality to hospital discharge, 28 (277) | ||||||||||||
| Survivors discharged home, 34/73 (46.6) | ||||||||||||
| Vail (2020) [48] | USA | Retrospective | 65,299 | 1,548 | 63,751 | 64.6±14.8 | 66.1±14.7 | 1,903 (53.2) | 168,833 (51.3) | Not mentioned | At least one charge for high-dose IV ascorbic acid along with at least one charge for both IV hydrocortisone and IV thiamine at any dose | Mortality for hospital, 437 (28.2) |
| Mohamed (2020) [49] | India | RCT | 85 | 45 | 43 | 58.7±14.9 | 59.4±15 | 31 (69) | 32 (74) | Standard of care for septic shock with hydrocortisone and vitamin supplements according to the treating physician’s discretion | Ascorbic acid 1.5 g IV every 6 hours for 4 days; hydrocortisone 50 mg IV every 6 hours for 4 days; thiamine 200 mg IV every 12 hours for 4 days | Mortality, 23/43 (53) |
| Time to shock reversal, 34.58±22.63 hours | ||||||||||||
| Hospital LOS, 20.9±15.01 days | ||||||||||||
| Long (2020) [50] | USA | Retrospective | 206 | 79 | 127 | 64.4±13.9 | 61.1±16.2 | 43 (54.4) | 70 (55.9) | 2012 Sepsis Guidelines including 30 mL/kg of crystalloid, broad spectrum antibiotics, and vasopressor therapy; steroids at the discretion of the physician | Ascorbic acid 1.5 g IV every 6 hours; hydrocortisone 50 mg IV every 6 hours; thiamine 200 mg IV every 12 hours | Hospital mortality, 21 (26.6) |
| ICU mortality, 9 (11.4) | ||||||||||||
| Vasopressor duration (median), 13.9 hours | ||||||||||||
| RRT initiation, 11/74 (14.9) | ||||||||||||
| Ventilator duration, 3.4 days | ||||||||||||
| ICU LOS (median), 2.0 days | ||||||||||||
| Hospital LOS (median), 9.5 days | ||||||||||||
| Chang (2020) [51] | China | RCT | 80 | 40 | 40 | 59.5±15 | 63.7±12.8 | 22 (57.5) | 21 (52.5) | 2016 International management of sepsis guidelines including resuscitation, antimicrobial therapy, vasopressor strategy, mechanical ventilation, and RRT | Ascorbic acid 1.5 g IV every 6 hours for 4 days or until ICU discharge; hydrocortisone 50 mg every 6 hours for 7 days or until ICU discharge; thiamine 200 mg IV every 12 hours for 4 days or until ICU discharge | 28-Day mortality, 11 (27.5) |
| ICU LOS, 7.5 days (4–12.8) | ||||||||||||
| Duration of vasopressors, 46 hours (23.8–102.5) | ||||||||||||
| New AKI, 1 (2.5) | ||||||||||||
| Delta SOFA score (72 hours), 3.5±3.3 | ||||||||||||
| Procalcitonin clearance (72 hours), 75.8 (62.2–86.4) | ||||||||||||
| Duration of mechanical ventilation, 126.5 hours (63.5–239.3) | ||||||||||||
| Lactate clearance (72 hours), 21.3% (–49.7% to 44.2%) | ||||||||||||
| Sevransky (2021) [52] | USA | RCT | 501 | 252 | 249 | 60.6±13.4 | 61±16.4 | 139 (55.2) | 134 (53.8) | Matching placebo | Ascorbic acid 1.5 g, thiamine 100 mg, hydrocortisone 50 mg every 6 hours; patients can be given corticosteroids. | Ventilator- and vasopressor-free days, 25 (0–29) |
| Mortality at 30 days, 56 (22.2) | ||||||||||||
| ICU mortality, 52 (20.6) | ||||||||||||
| Mortality at 180 days, 102 (40.5) | ||||||||||||
| Length of ICU stay, 6.7 (7.3) | ||||||||||||
| Length of hospital stay, 12.6 (10) | ||||||||||||
Values are presented as mean±standard deviation, number (%), or median (interquartile range) unless otherwise indicated.
IV: intravenous; ICU: intensive care unit; LOS: length of stay; RRT: renal replacement therapy; AKI: acute kidney injury; VP: vasopressors; MV: mechanical ventilation; RCT: randomized controlled trial; SOFA: Sequential Organ Failure Assessment.




 PDF
PDF Citation
Citation Print
Print



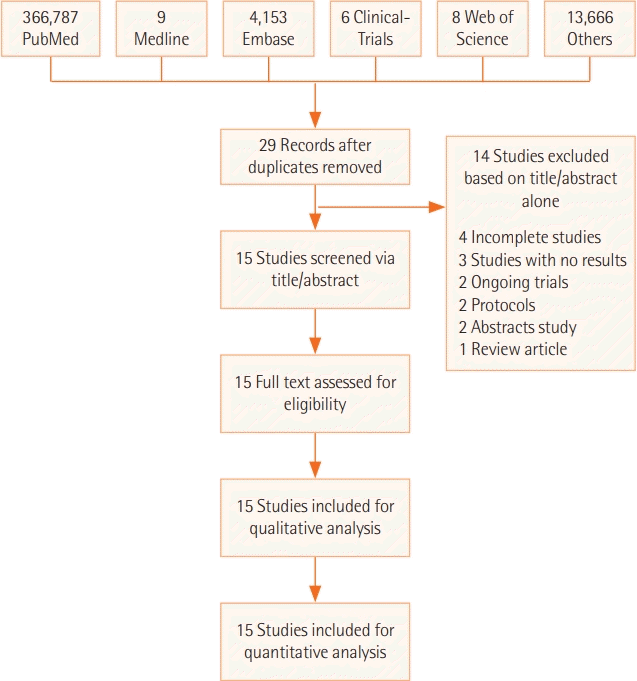
 XML Download
XML Download