Abstract
The most common cardiac cause of massive hemoptysis is mitral stenosis. Mitral regurgitation is rarely complicated by massive hemoptysis. A 48-year-old man with no significant medical history was admitted to our hospital with hemoptysis and production of 500 mL of blood within 24 hours. A pan-systolic murmur was found on chest examination. A chest computed tomography showed airspace consolidation in the right upper and middle lobes, with faint bilateral ground glass opacity. Echocardiography revealed mitral valve prolapse and grade IV mitral regurgitation. The patient was diagnosed with sporadic primary mitral valve prolapse. After mitral valve repair surgery, the patient recovered fully.
There are many conditions that may be complicated by massive hemoptysis, including pulmonary tuberculosis, bronchiectasis, necrotizing pneumonia, aspergilloma, and lung neoplasm.[1,2] Cardiovascular conditions that lead to pulmonary venous hypertension can cause cardiac hemoptysis. In most instances, pulmonary edema will occur bilaterally; in rare cases, focal imbalance leads to the development of unilateral or lobar edema. The most common cardiac-related cause of massive hemoptysis is mitral stenosis, while mitral regurgitation (MR) rarely results in this condition.[3-6] In addition, most MR-related cases of hemoptysis are associated with traumatic injury while non-traumatic causes are seldom reported.[7-11] Here, we present a case study of acute MR and mitral valve prolapse with massive hemoptysis and unilateral consolidation of the right lung.
A 48-year-old man was admitted to our hospital with acute onset of massive hemoptysis. The patient presented with 3-4 occurrences of hemoptysis (total volume > 500 mL) within a 24-hour period, accompanied by a cough and dyspnea. Past medical and drug history was nonspecific, with the exception of his status as a smoker and his employment in the textile processing industry.
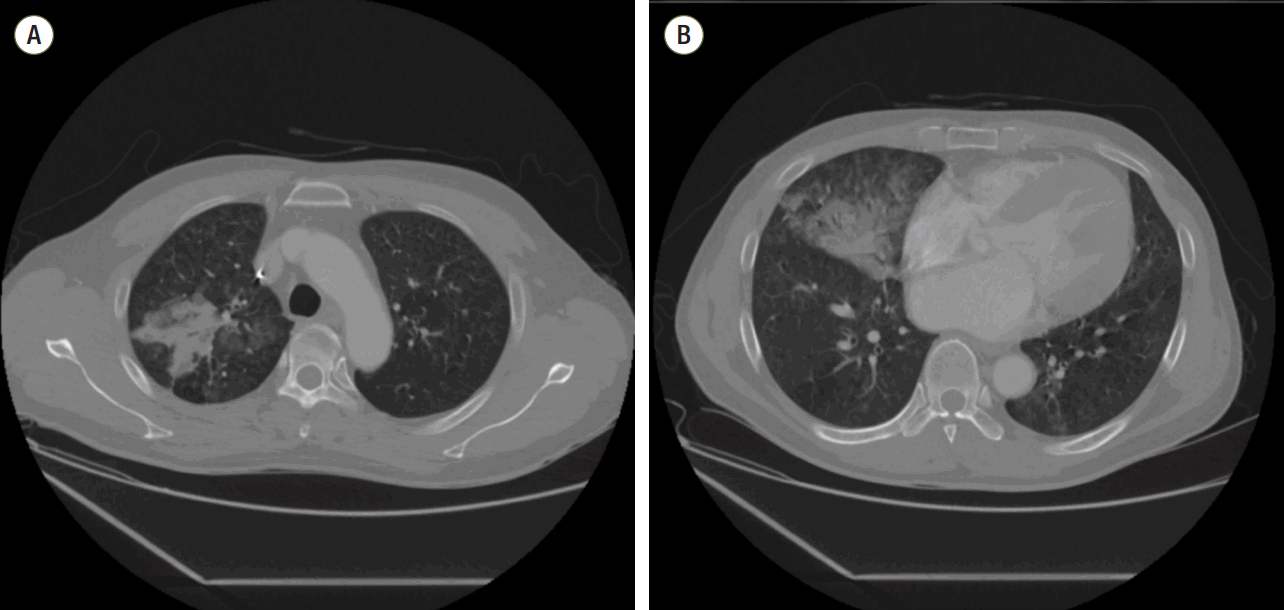
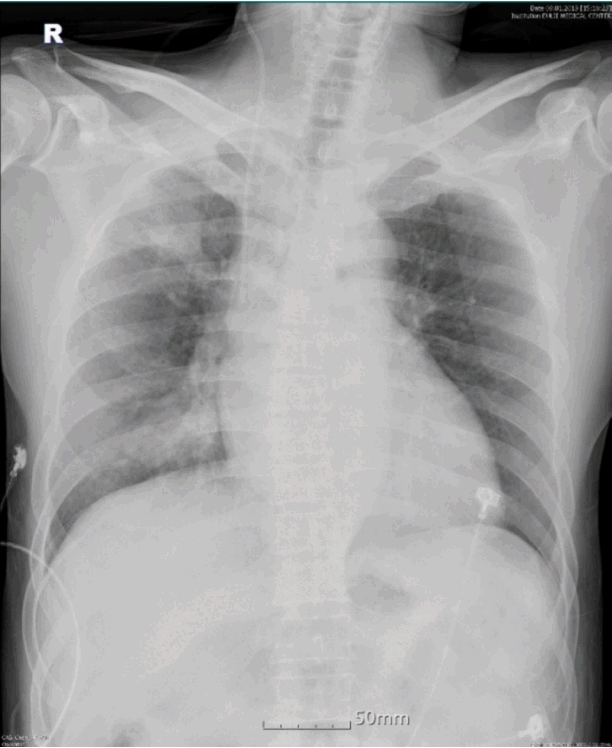
Vital signs were as follows: blood pressure, 235/155 mmHg; respiratory rate, 20/min; pulse rate, 104/min; and body temperature, 36.5℃. On chest auscultation, crackles were audible in the right lung field and a 3/6-pansystolic murmur was best heard over the cardiac apex. Chest radiograph showed mild cardiomegaly and consolidations on the right lung (Fig. 1). Chest computed tomography (CT) showed airspace consolidations in the right upper and middle lobes with faint, bilateral ground glass opacity and left atrial enlargement (Fig. 2). Arterial blood gas analysis revealed a PaO2 of 76 mmHg with an oxygen saturation of 95%. Initial hemoglobin was 16.1 g/dL, white blood cell count was 8,800/mm3 (neutrophils, 82.6%; eosinophils, 0.9%) and platelet count was 180,000/mm3.[5] The erythrocyte sedimentation rate was 2 mm/hr and C-reactive protein was 0.27 mg/dL, while BNP was 156 pg/mL and N-terminal prohormone of brain natriuretic peptide was 395.8 pg/mL. Prothrombin time was 11.7 sec (international normalized ratio 1.11) and partial thromboplastin time was 33.8 sec. The patient was started on intravenous administration of ceftriaxone (2 g/day) with tranexamic acid (1,500 mg/day) as an antifibrinolytic for presumed community-acquired pneumonia complicated with hemoptysis. Hemoptysis subsided gradually over the first 6 hr post-admission, although intermittent expectoration of blood-tinged sputum persisted.
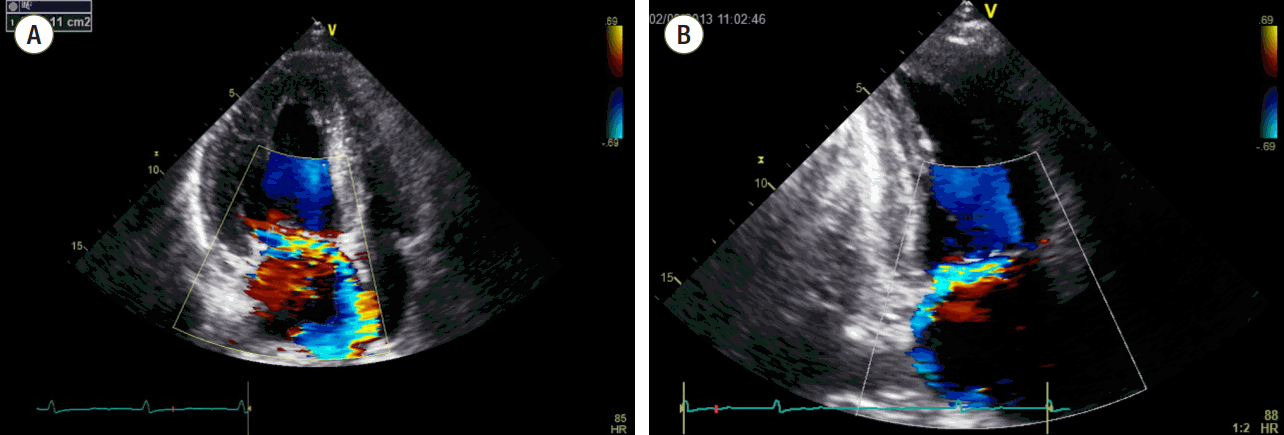
On the second day post-admission, fiberoptic bronchoscopy revealed a pervading blood clot in the right upper lobar bronchus extending to the posterior segmental bronchus of the right upper lobe. Bronchoalveolar lavage was performed in the right upper lobe and bloody fluid was persistently retrieved, suggesting alveolar hemorrhage. Microbiological studies included blood and sputum culture, sputum acid-fast stain, tuberculosis polymerase chain reaction, and mycoplasma and HIV antibodies, which were all negative. Transthoracic echocardiography showed an ejection fraction of 58% with normal ventricular size (left ventricular end-systolic dimension end-systolic dimension 35mm, LV end-diastolic dimension 51mm), an enlarged left atrium (left atrial volume index 42.3 mm3/m2), and severe mitral regurgitation (Grade IV) with significant prolapse of the mitral valve (P1). Interestingly, estimated pulmonary artery systolic pressure was within the normal range (right ventricular pressure 23 mmHg), with minimal aortic and tricuspid regurgitation. The E/e’ could not be calculated due to severe mitral regurgitation and no regional wall motion abnormality was observed (Fig. 3).
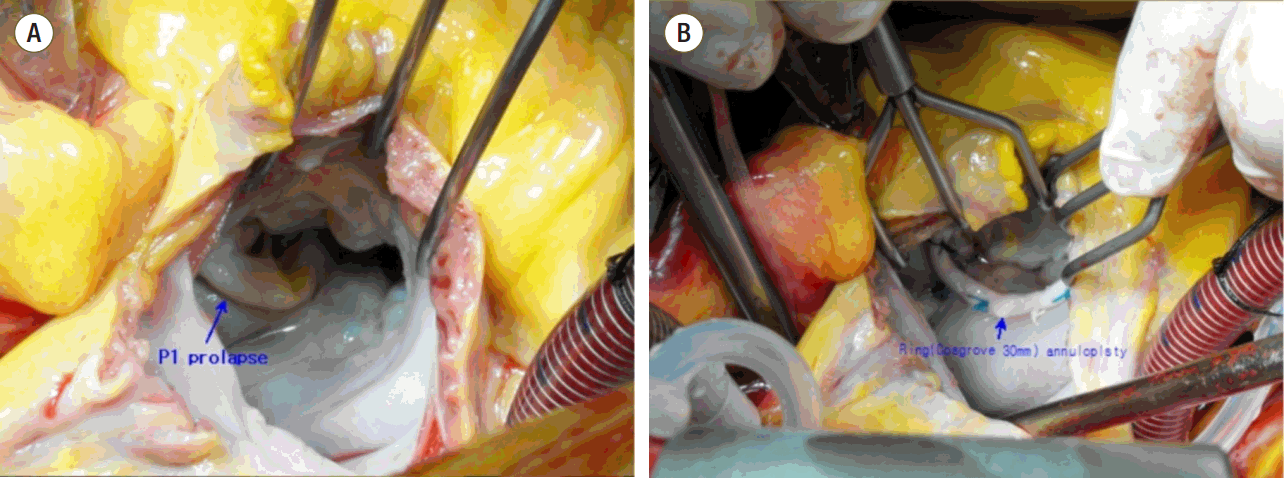
On hospital day 5, coronary angiography showed 40% stenosis in the left anterior descending and right coronary arteries, but there was no need for coronary angioplasty. On hospital day 10, open-heart valvuloplasty of the mitral valve was performed and showed prolapse of the relatively thick and mildly calcified P1 scallop of the mitral valve (Fig. 4). Pathological finding of the P1 scallop revealed fibrosis and myxomatous infiltration, which was thought to be the cause of the observed mitral valve prolapse. A transthoracic echocardiogram post-procedure found no evidence of mitral regurgitation and the patient recovered fully from hemoptysis and associated radiographic abnormalities.
Most cases of alveolar hemorrhage from pulmonary causes are associated with capillary inflammation. In contrast, the pathogenesis of alveolar hemorrhage from cardiac causes appears to be attributable to mechanical pressure overload of the capillary. Acute MR may result in a regurgitant jet directed towards the right upper pulmonary vein and this may result in right-side pulmonary edema and alveolar hemorrhage.[9]
The group Kim et al.[8] reported a case of diffuse ground glass opacity on a simple chest radiograph with no significant bleeding of the lesions upon bronchoscopic examination. While the patient was being treated for pneumonia, hemoptysis ceased on hospital day 7 before recurring on hospital day 14. MR due to chordae rupture was observed on re-evaluation and the patient was treated with emergency surgery. The fact that there was diffuse alveolar hemorrhage with no significant bleeding lesions on the fiberoptic bronchoscopy showed a different view to that presented in our case. Marak et al.[9] reported a case of mitral valve prolapse suggesting vague infiltration in a simple chest radiograph. However, there was no incidence of hemoptysis although blood was observed during bronchial washing by bronchoscopy. However, at the follow-up, persistent hemoptysis with decreased oxygen saturation was observed; ultimately, emergent mitral valve repair was performed.
In contrast to previous case reports, our case showed consolidation in the right upper and middle lobes, a radiographic finding most often seen in cases of bacterial pneumonia. Hemoptysis and an obvious murmur on auscultation – rather than specific signs of inflammation, such as fever or leukocytosis – is an early sign of a cardiac cause for hemoptysis. We were able to find the bleeding lesion through bronchoscopy, which appeared as a consolidation on the CT scan, and further confirmed alveolar hemorrhage by observing a persistently pinkish color in the retrieved bronchoalveolar lavage fluid.
In this patient, MR caused by increased mitral valve motion is classified as Type II according to Carpentier’s surgical classification of mitral valve pathology. The most common etiology of this type of MR is myxomatous infiltration of the valve with consequent mitral valve prolapse. Other causes include ischemic MR, Marfan’s syndrome, Ehlers-Danlos syndrome, traumatic MR, endocarditis, and acute rheumatic myocarditis.[12] A multicenter study in Korea reported that mitral valve prolapse due to myxomatous degeneration appeared to occur most frequently in the A3 scallop.[13] In patients with mitral valve regurgitation who have undergone open-heart surgery, myxomatous degeneration appears more frequently in P3 before the A3 scallop.[14] Myxomatous degeneration in cardiac valves occurs most frequently in the A3 scallop, although in the present case this occurred in the P1 scallop. In this patient, estimated pulmonary artery systolic pressure showed normal range. It was explained mean right ventricular afterload wasn’t influenced by MR, because regurgitant jet flow limited in right upper pulmonary vein. Some serologic studies would be useful to distinguish other causes of mitral regurgitation. However, there were no physical findings to suggest vasculitis and connective tissue disease. The patient fully recovered after valvuloplasty of mitral valve. Thus, we were able to diagnose the patient as having sporadic primary mitral valve prolapse, as he had no personal or familial history of heart disease, connective tissue disorder, or previous trauma.
The localized right upper lobar bleeding observed in this case may be attributable to the uneven distribution of regurgitant jet flow. In previous studies, 8% of MR presents with unilateral edema with all such cases developing in the right lung.[5] Pulmonary edema and right upper lobar consolidation can be explained by the anatomical features of the pulmonary vein. Regurgitant jet flow can cause a relatively higher hydrostatic pressure in the right upper and middle lobe pulmonary veins than in the right lower lobe.[15,16] Combined consolidation in the right upper middle lobes, which was observed in this case, can be explained by drainage of the right middle lobar pulmonary vein into the right upper lobar pulmonary vein.
The present case suggests that acute MR with sporadic primary mitral valve prolapse can be a cause of alveolar hemorrhage with unilateral pulmonary consolidation. In patients with hemoptysis and unilateral consolidation, careful physical examination and cardiac evaluation (such as echocardiography) may assist an early diagnosis. Surgical repair of the mitral valve should be considered, as hemoptysis may aggravate the condition resulting in relapse.
References
1. Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration. 2010; 80:38–58.

2. Fartoukh M, Khalil A, Louis L, Carette MF, Bazelly B, Cadranel J, et al. An integrated approach to diagnosis and management of severe haemoptysis in patients admitted to the intensive care unit: a case series from a referral centre. Respir Res. 2007; 8:11.

3. Woolley K, Stark P. Pulmonary parenchymal manifestations of mitral valve disease. Radiographics. 1999; 19:965–72.

4. Roach JM, Stajduhar KC, Torrington KG. Right upper lobe pulmonary edema caused by acute mitral regurgitation. Diagnosis by transesophageal echocardiography. Chest. 1993; 103:1286–8.
5. Schnyder PA, Sarraj AM, Duvoisin BE, Kapenberger L, Landry MJ. Pulmonary edema associated with mitral regurgitation: prevalence of predominant involvement of the right upper lobe. AJR Am J Roentgenol. 1993; 161:33–6.

6. Yoon YG, Bang DS, Park BC, Lee SH, Kim JS, Park Y, et al. Localized pulmonaryeEdema in patient with severe mitral regurgitation. Tuberc Respir Dis. 2005; 59:432–5.
7. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 17-1995. An 81-year-old woman with mitral regurgitation and a left-upper-lobe pulmonary infiltrate. N Engl J Med. 1995; 332:1566–72.
8. Kim U, Hong GR, Kim DH, Lee SH, Park JS, Shin DG, et al. Diffuse alveolar hemorrhage due to acute mitral tegurgitation. J Cardiovasc Ultrasound. 2007; 15:16–8.
9. Marak CP, Joy PS, Gupta P, Bukovskaya Y, Guddati AK. Diffuse alveolar hemorrhage due to acute mitral valve regurgitation. Case Rep Pulmonol. 2013; 2013:179587.

10. Spence TH, Connors JC. Diffuse alveolar hemorrhage syndrome due to ‘silent’ mitral valve regurgitation. South Med J. 2000; 93:65–7.

11. Yeung AWT, Lau ACW, Shum HP, Lam GSM, Chan KKC, Li SK, et al. Diffuse alveolar hemorrhage and intravascular hemolysis due to acute mitral valve regurgitation. Crit Care Shock. 2013; 16:3–7.
12. de Marchena E, Badiye A, Robalino G, Junttila J, Atapattu S, Nakamura M, et al. Respective prevalence of the different carpentier classes of mitral regurgitation: a stepping stone for future therapeutic research and development. J Card Surg. 2011; 26:385–92.

13. Song JK, Song JM, Kim YJ, Kang SJ, Kang DH, Chae SC, et al. Lesion characteristics of mitral valve prolapse due to myxomatous degeneration in Korea: a prospective multicenter study using echocardiography. Korean Circ J. 2005; 35:904–9.

14. Kang SJ, Song JK, Kim HS, Song JM, Kang DH, Lee CW, et al. Clinical characteristics of surgically corrected mitral regurgitation due to myxomatous degeneration in Korea. Korean Circ J. 2001; 31:1042–8.

Fig. 2.
The transverse (A, B) images of the chest computed tomograhphy show patchy consolidation in the right upper and middle lobes and bilateral ground glass opacity with no mass.




 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download