Abstract
Severe sepsis and septic shock are the main causes of death in critically ill patients. Early detection and appropriate treatment according to guidelines are crucial for achieving favorable outcomes. Endotoxin is considered to be a main element in the pathogenic induction of gram-negative bacterial sepsis. Polymyxin B hemoperfusion can remove endotoxin and is reported to improve clinical outcomes in patients with intra-abdominal septic shock, but its clinical efficacy for pneumonic septic shock remains unclear. Here, we report a case of a 51-year-old man with pneumonic septic shock caused by Pseudomonas aeruginosa, who recovered through polymyxin B hemoperfusion.
Severe sepsis or septic shock leads to considerable morbidity and mortality.[1,2] Endotoxin, an outer membrane component of gram-negative bacteria, plays an important role in the pathogenesis of severe sepsis or septic shock, with resulting poor clinical outcomes.[3,4] Because of this, there has been a focus on treatment by removing endotoxin from the blood.[5] Polymyxin B, a cationic polypeptide antibiotic, has a strong affinity for endotoxin and is able to bind the lipid A portion of endotoxin through ionic and hydrophobic interactions.[6] This property has led to the development of polymyxin B-immobilized fiber column hemoperfusion (Toraymyxin 20-R; Toray Industries, Inc, Tokyo, Japan).[7] However, although polymyxin B hemoperfusion has proved to be effective in treating intra-abdominal septic shock,[8] evidence of its effectiveness in treating pneumonia septic shock is still lacking.
Here, we report a clinical case of a patient who recovered from refractory pneumonic septic shock caused by Pseudomonas aeruginosa after treatment with polymyxin B-immobilized fiber column hemoperfusion.
A 51-year-old man presented to a hospital emergency department with a one-day history of fever and nausea. He had been diagnosed with advanced stage non-small cell lung cancer six months previously and had received cyclic chemotherapy seven days before presentation. A physical examination revealed a blood pressure (BP) of 126/76 mmHg; pulse rate (PR) of 131 beats/min; respiratory rate (RR) of 24 breaths/min; temperature of 38.9°C; and oxygen saturation of 99% on room air. According to the initial laboratory test results, the peripheral blood absolute neutrophil count was 490/mm3. Creactive protein and procalcitonin levels were 2.24 mg/dL (normal reference <0.6 mg/dL) and 21.29 ng/mL (normal reference <0.5 mg/dL), respectively. The results of initial arterial blood gas analysis were as follows: pH: 7.480, partial arterial carbon dioxide pressure: 19 mmHg, partial arterial oxygen pressure: 77 mmHg, bicarbonate: 14 mmol/L, and lactic acid: 3.9 mmol/L.
Initial chest radiography showed increased opacity in the right upper lung zone compared to a previous chest radiograph (Fig. 1). After diagnosis of pneumonia accompanied by febrile neutropenia, sputum and blood cultures were performed and intravenous piperacillin/tazobactam 4500 mg every 6 hours and levofloxacin 750 mg every 24 hours were administered as empirical broad spectrum antibiotics. Six hours later, the patient’s vital signs had changed: his BP was 89/58 mmHg; PR, 179 beats/min; and RR, 45 breaths/min. Despite adequate infusion of intravenous fluid, shock persisted and vasopressors were started. The patient was transferred to the medical intensive care unit (ICU). On admission to the ICU, the patient was intubated and mechanically ventilated due to progression of hypoxemia. Vasopressors including norepinephrine, vasopressin, and epinephrine were administered and corticosteroids were also infused continuously. On the second day of ICU admission, gram negative bacteria were identified in the patient’s blood culture and he was still hemodynamically unstable. Following diagnosis of refractory septic shock caused by gram negative bacteria, treatment using polymyxin B hemoperfusion was decided on. Two sessions of polymyxin B hemoperfusion in addition to standard medical therapy were planned.
Polymyxin B hemoperfusion was performed according to the following method: an adsorbent column containing 5 mg of polymyxin B per gram of polystyrene fiber (Toraymyxin 20-R, Toray Industries, Inc, Tokyo, Japan; Toraymyxin 20-R PMX column, Toray Industries, Tokyo, Japan) was washed by perfusion with 4 L normal saline. After inserting a double-lumen catheter into a femoral vein, blood was drawn from the proximal port and perfused through the distal port of the catheter. Although unfractionated heparin is commonly used as an anticoagulant, the blood was perfused at a rate of 80 to 100 m/min using the protease inhibitor nafamostat mesilate (Torii Pharmaceuticals, Co, Ltd, Tokyo, Japan) in this case.[9] Polymyxin B hemoperfusion was maintained for more than 2 hours using a renal replacement machine. After the first session of hemoperfusion, hemodynamic instability continued and the vasopressor dose was not reduced. Echocardiography was performed and stress-induced cardiomyopathy was detected. The second session of polymyxin B hemoperfusion was performed 24 h later. After this, the patient stabilized and the vasopressor dose was decreased (Table 1). Four days later, blood cultures revealed Pseudomonas aeruginosa infection, which was susceptible to initial empirical antibiotics. The patient was extubated seven days after the second session of polymyxin B hemoperfusion. His condition improved and he was transferred to the general ward eight days after the second session of polymyxin B hemoperfusion. He was discharged 10 days after that.
Severe sepsis and septic shock are common causes of death in an ICU.[1,2] Early recognition and management are important for improving clinical outcomes.[10] Endotoxin is an outer membrane component of gram negative bacteria and plays a significant role in the pathogenesis of severe sepsis or septic shock.[3] High levels of endotoxin activity are associated with worse clinical outcomes.[4] Therefore, attention has been paid to the removal of endotoxin.[5] Polymyxin B, a cationic polypeptide antibiotic, has a strong affinity for endotoxin and is able to bind the lipid A portion of endotoxin through ionic and hydrophobic interactions.[6] Based on this binding property, polymyxin B-immobilized fiber column hemoperfusion was developed to remove the circulating endotoxin in blood.[7] Leukocyte or platelet counts may decrease after polymyxin B hemoperfusion, but significant adverse effects have not been reported.[11,12]
Ronco and Klein[12] recently described the mechanism of polymyxin B hemoperfusion. This mainly relies on direct adsorption of circulating lipopolysaccharide (LPS), which depends on both the ability of the polystyrene-polymyxin B to bind and the LPS-polymyxin B affinity. A secondary mechanism is probably the removal of activated inflammatory cells such as monocytes and neutrophils. The authors also summarized polymyxin B hemoperfusion clinical data. In the EUPHAS trial, Cruz DN et al.[13] showed that early use of polymyxin hemoperfusion therapy in treating abdominal septic shock, when added to conventional medical therapy, improved clinical outcomes including hemodynamics and organ dysfunction. This approach reduced 28-day mortality due to severe sepsis and septic shock caused by intra-abdominal gram negative infections.[8] Several meta-analyses also reported the clinical benefits of polymyxin B hemoperfusion in improving hemodynamics and reducing mortality in patients with septic shock.[13-15] Clinical data from Early Use of Polymyxin B Hemoperfusion in Abdominal Septic Shock 2 (EUPHAS2) and ABDO-MIX is expected to be released in the future. The Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized Controlled Trial of Adults Treated for Endotoxemia and Septic Shock (EUPHRATES) trial (evaluating the use of polymyxin B hemoperfusion in a randomized controlled trial of adults treated for endotoxemia and septic shock), a multicentered, blinded, randomized controlled trial of polymyxin B hemoperfusion in patients with septic shock, is also being conducted.
Polymyxin B hemoperfusion is considered a standard treatment for septic shock in Japan;[9] however, in South Korea, it is not available as an adjunctive therapy to treat septic shock. Clinical data on the technique are insufficient. Furthermore, the role of polymyxin B hemoperfusion in treating pneumonic septic shock has not been established.
In our case, septic shock was caused by pneumonia accompanied by febrile neutropenia. We managed a patient with maximal conventional therapy according to guidelines. [10] Despite these efforts, the patient’s clinical condition worsened. Blood cultures revealed gram negative bacteria. The prognosis was poor due to both his advanced lung cancer for which he was receiving systemic chemotherapy seven days before admission and the high dose vasopressor drugs. Although there is not much evidence of the effectiveness of polymyxin B hemoperfusion, this was applied it this patient and a good clinical outcome was achieved. The level of endotoxin was not measured in this case because endotoxin assays are not available as a routine practice in our center. On the basis of our experience with this case, it is difficult to make a generalized recommendation for polymyxin B hemoperfusion in all patients with pneumonic septic shock and more studies will be needed to clarify whether polymyxin B hemoperfusion is effective.
In conclusion, our case report highlights that polymyxin B hemoperfusion might be a good adjunctive therapy in the treatment of refractory pneumonic septic shock caused by gram negative bacteria.
References
1. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Eng J Med. 2003; 348:1546–54.

2. Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007; 35:1244–50.

3. Danner RL, Elin RJ, Hosseini JM, Wesley RA, Reilly JM, Parillo JE. Endotoxemia in human septic shock. Chest. 1991; 99:169–75.

4. Marshall JC, Foster D, Vincent JL, Cook DJ, Cohen J, Dellinger RP, et al. Diagnostic and prognostic implications of endotoxemia in critical illness: results of the MEDIC study. J Infect Dis. 2004; 190:527–34.

6. Zavascki AP, Goldani LZ, Li J, Nation RL. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother. 2007; 60:1206–15.

7. Shoji H, Tani T, Hanasawa K, Kodama M. Extracorporeal endotoxin removal by polymyxin B immobilized fiber cartridge: designing and antiendotoxin efficacy in the clinical application. Ther apher. 1998; 2:3–12.

8. Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009; 301:2445–52.
9. Mitaka C, Fujiwara N, Yamamoto M, Toyofuku T, Haraguchi G, Tomita M. Polymyxin B-immobilized fiber column hemoperfusion removes endotoxin throughout a 24-hour treatment period. J Crit Care. 2014; 29:728–32.

10. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013; 41:580–637.

11. Shoji H. Extracorporeal endotoxin removal for the treatment of sepsis: endotoxin adsorption cartridge (Toraymyxin). Ther Apher Dial. 2003; 7:108–14.
12. Ronco C, Klein DJ. Polymyxin B hemoperfusion: a mechanistic perspective. Crit Care. 2014; 18:309.

13. Cruz DN, Perazella MA, Bellomo R, de Cal M, Polanco N, Corradi V, et al. Effectiveness of polymyxin B-immobilized fiber column in sepsis: a systematic review. Crit Care. 2007; 11:R47.

14. Mitaka C, Tomita M. Polymyxin B-immobilized fiber column hemoperfusion therapy for septic shock. Shock. 2011; 36:332–8.

15. Zhou F, Peng Z, Murugan R, Kellum JA. Blood purification and mortality in sepsis: a meta-analysis of randomized trials. Crit Care Med. 2013; 41:2209–20.
Fig. 1.
Compared to previous chest radiography results (A), greater opacity in the right upper lung field was revealed (B). L: left; R: right.
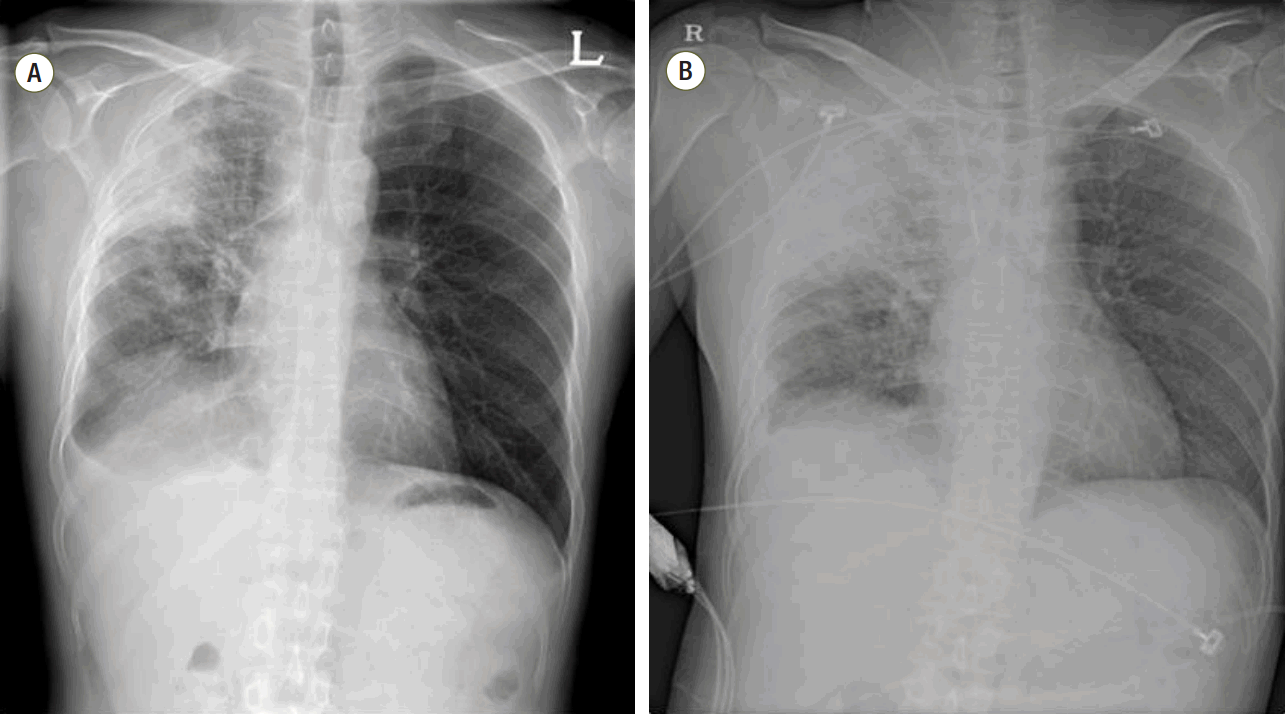
Table 1.
Serial follow-up of mean arterial pressure, oxygenation, arterial lactate and dosage of vasopressors before, during and after polymyxin B hemoperfusion




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download