Abstract
Purpose
The purpose of this study was to estimate the incidence of occult gastrointestinal (GI) primary tumours in patients with metastatic cancer of uncertain primary origin and evaluate their influence on treatments and overall survival (OS).
Materials and Methods
We used population heath data from Manitoba, Canada to identify all patients initially diagnosed with metastatic cancer between 2002 and 2011. We defined patients to have “occult” primary tumour if the primary was found at least 6 months after initial diagnosis. Otherwise, we considered primary tumours as “obvious.” We used propensity-score methods to match each patient with occult GI tumour to four patients with obvious GI tumour on all known clinicopathologic features. We compared treatments and 2-year survival data between the two patient groups and assessed treatment effect on OS using Cox regression adjustment.
Results
Eighty-three patients had occult GI primary tumours, accounting for 17.6% of men and 14% of women with metastatic cancer of uncertain primary. A 1:4 matching created a matched group of 332 patients with obvious GI primary tumour. Occult cases compared to the matched group were less likely to receive surgical interventions and targeted biological therapy, and more likely to receive cytotoxic empiric chemotherapeutic agents. Having an occult GI tumour was associated with reduced OS and appeared to be a nonsignificant independent predictor of OS when adjusting for treatment differences.
According to the Canadian Cancer Society, gastrointestinal (GI) cancer is the most common type of cancer and cause of cancer death in Canada [1]. In 2016, 46,000 Canadians will be diagnosed with cancers of the GI tract involving the esophagus, stomach, biliary system, pancreas, small intestine, colon, rectum, and anus and 22,000 Canadians will die from these cancers [1]. The tendency of GI cancers to be either asymptomatic at early stages or to present with vague symptoms that might be mistaken for other inflammatory diseases at more advanced stages, as well as the lack of accurate screening procedures for many of these cancers, contribute to the diagnosis of GI cancers at more advanced-stages, often after they metastasize to other areas of the body [2].
Patients with metastatic cancers of the GI tract may have clinical and pathologic presentation masking their actual GI tract origin (i.e., occult GI tumour). A series of recent analyses of gene profiling molecular assays predicted the GI tract to be the most common cancer site of origin in patients initially diagnosed with metastatic cancer of unknown origin, accounting for 34% to 45% of these patients [3-5]. In a small number of case reports, metastatic disease of occult GI primary tumours have also been shown to have clinicopathologic features that mimic metastatic disease from other cancer sites of origin leading to a diagnostic and thus treatment conundrum [6-14]. It is essential to correctly distinguish a GI primary site from metastatic disease of other primary sites not only for selection of a growing arsenal of effective first-line site-specific or targeted therapies which may improve survival [15], but also for the selection of secondline chemotherapy, decisions regarding debulking surgery or surgery for resection of metastases, optimal management of symptoms, prognosis, and recommendations regarding entry into hospice care.
Currently, little is known about the clinical significance of metastatic disease of occult GI primary tumours in actual clinical practice. In this study, we used provincial heath administrative databases in the Canadian province of Manitoba to identify occult GI primary tumours in patients with metastatic cancer of uncertain primary (i.e., difficult to diagnose primary) and estimate their actual incidence. We also aimed to compare those patients to their counterparts of patients with metastatic disease of obvious (i.e. readily diagnosed) GI primary tumours to evaluate the impact of having an occult GI primary tumour on disease management and overall survival.
We conducted a retrospective cohort study using administrative health data obtained by linking the databases of the Manitoba Cancer Registry (MCR) and Provincial Pharmacy program of CancerCare Manitoba (CCMB) with Manitoba Health’s administrative databases, including the Hospital Discharge Database, Physician Claims Database and the Drug Program Information Network (DPIN). A full description of these databases, their contents and the linkage process has been reported elsewhere [16-19].
We used the MCR to identify all metastatic cancer patients (defined as stage IV or distant metastasis within 4 months of initial diagnosis) during the period from January 1, 2002, to December 31, 2011. All Manitoba residents aged 18 to 90 years old with no history of cancer at diagnosis who had their metastatic disease histologically confirmed and survived at least 6 months following their initial cancer diagnosis were eligible for inclusion in our metastatic patient population. This 6-month window was important to ensure that patients would have had reasonable survival time during the early course of their metastatic disease to undergo a full diagnostic workup and have their primary tumour site identified [20]. When the primary tumour was identified 6 months or more after initial diagnosis, we defined patients to have “occult” primary tumour (i.e., metastatic cancer of uncertain primary). Otherwise, we defined patients to have “obvious” primary tumour. Our case definition is consistent with other attempts at identifying occult primary tumours [20]. Full details regarding the identification of our metastatic patient population are reported elsewhere [21].
For this analysis, we used our metastatic patient population to identify all patients diagnosed with metastatic cancer of GI sites including esophagus, gastroesophageal junction, stomach, small intestine, colon, rectum, anus, anal canal, liver, intra hepatic bile duct, extra hepatic bile duct, gallbladder, pancreas, and other unspecified GI tract. We stratified this group into two main subgroups: (1) patients with occult GI tumours and (2) patients with obvious GI tumours. Two-year follow-up information was collected from the MCR for each patient in the two subgroups including surgical and therapeutic radiology procedures, systemic therapies, palliative care, diagnosis of second primary and death.
We linked those patients with the Provincial Pharmacy Program of CCMB and Manitoba Health’s administrative databases to validate all cancer therapy data captured by the MCR; to collect additional information on types of radiotherapy, chemotherapy, and targeted cancer therapy agents as described elsewhere [16-19]; and to measure co-morbidity using the method developed by Charlson et al. [22] and used elsewhere [18,19,21]. We also used, in particular, the Physician Claims Database to collect information on GI diagnostic examinations received during the diagnostic workup (defined as the period from 6 months before to 6 months after cancer diagnosis) for all identified patients diagnosed with metastatic cancer of GI sites. The GI diagnostic examinations recorded in the Physician Claims Database included diagnostic laparoscopy or laparotomy, diagnostic GI endoscopic examinations (i.e., esophagoscopy and esophagogastroduodenoscopy, gastroscopy, enteroscopy, endoscopic ultrasound, colonoscopy, and proctosigmoidoscopy), taking of biopsy from a GI site, abdominal ultrasound, computerized axial tomography (CT) scan of the abdomen, magnetic resonance imaging (MRI) scan of the abdomen, and GI nuclear scans.
Ethics approval for the study was obtained from the University of Manitoba Health Research Ethics Board, Manitoba Health Information Privacy Committee and University of Western Ontario Health Research Ethics Board.
Continuous data are reported as mean±standard deviation, and categorical data, as numbers and percentages. Categorical data were compared using the chi-square test. Quantitative variables were compared using the t test. All statistical tests were two-sided and results were considered significant at the 5% critical level. Statistical analysis was performed using SAS ver. 9.3 (SAS Institute Inc., Cary, NC).
We performed a matched analysis within our cohort study. We used logistic regression to create a propensity score (i.e., likelihood) [23] for having occult GI primary tumour, using the following potential confounders, which were available for the entire cohort: age, sex, Charlson co-morbidity score, number and type of metastatic sites, grade differentiation, primary tumour site, histology, and year of initial diagnosis, regardless of their individual statistical significance. We used the propensity score to match each patient who had an occult GI tumour with up to four patients who had obvious GI tumour on the estimated propensity score. To avoid a poor quality match, we only considered observations that were within a ±0.01 of the occult unit’s propensity score for matching and chose the closest match without replacement (i.e., caliper matching without replacement) [23]. When no matches were found that case would be dropped.
Time to death was assessed using Kaplan-Meier survival curves. The curves were compared using the log-rank test statistic. Cox proportional hazard modeling was used to calculate hazard ratios (HRs) with associated 95% confidence intervals (CIs) to assess the differences between occult cases and the matched group with respect to 2-year overall survival (OS). We tested the effect of cancer treatments on the calculated HR for cases with occult versus matched patients with obvious GI primary tumours. We included receipt of surgical resection (no vs. yes), radiotherapy (no vs. yes), and systemic therapy (i.e., chemotherapy or biological targeted therapy) (no vs. yes) as covariates in a Cox proportional hazard model. We also tested the interactions between these covariates and status of primary tumour (occult vs. obvious GI tumour). In subgroup analyses in which we included patients from the case and matched groups who were treated with a given cancer therapy, we examined the effect of wait time after initial diagnosis to receive that cancer treatment and the effect of receipt of certain type of therapeutic agents versus others on OS.
In separate analyses we used Kaplan-Meier survival curves and Cox regression to compare the 2-year OS in the case group of patients with occult GI tumours to all patients with obvious GI primary tumours and generate HR. We conducted standard adjusted analyses by including all potential confounders mentioned earlier in this section as covariates in a Cox proportional hazard model. We also used the propensity score to adjust for differences in baseline characteristics between the two patient groups using two methods. First, we used the propensity score as a covariate in a Cox proportional hazard model and generate adjusted HR [23]. Second, we used a weighted Cox proportional hazards model and generate adjusted HR, where the weight assigned for each patient was based on the stabilized inverse propensity score as previously described [24].
There were 529 patients who had metastatic cancer of uncertain primary origin (i.e., had an occult primary tumour), accounting for 8.9% of all patients newly diagnosed with metastatic cancer who met the inclusion criteria (n=5,953) (Table 1). Of those, there were 83 patients with occult GI primary tumour, accounting for 15.7% of all patients with metastatic cancer of uncertain primary and 5% of all patients with metastatic GI primary tumour (n=1,656) (Table 1).
Prior to matching, patients with metastatic cancer of occult GI primary tumours presented with distinctive clinicopathologic features from their counterparts of all patients with obvious GI primary tumours (n=1,573) (Table 2). Using 1:4 matching on the estimated propensity score, we matched the case group of 83 patients with occult GI primary tumours with a group of 332 patients with obvious GI primary tumours. No occult cases were dropped due to poor match quality. Table 2 shows the baseline patient and tumour characteristics of the matched group (n=332) as compared to the case group of 83 patients with occult GI primary tumours. As a result of matching, we eliminated differences in age, sex, year of initial diagnosis, co-morbidity score, grade differentiation, GI primary tumour location, histology, and number and type of metastatic sites between occult cases and the matched group (Table 2). Compared to the matched group, occult cases experienced on average a longer time of 10.8 months after initial cancer diagnosis to have their primary tumour identified (Table 2). During the diagnostic workup, occult cases compared to the matched group received similar diagnostic laparoscopies or laparotomies and abdominal diagnostic imaging examinations. However, occult cases were less likely to receive any type of diagnostic GI endoscopic examinations (mean difference, 33.4%; 95% CI, 21.8 to 45; p < 0.001) including upper GI endoscopy (mean difference, 14.5%; 95% CI, 4.5 to 24.9; p=0.01), lower GI endoscopy (mean difference, 14.2%; 95% CI, 2.8 to 25.5; p=0.01), endoscopic ultrasound (mean difference, 15%; 95% CI, 10.5 to 19.7; p < 0.001), and endoscopic retrograde cholangio-pancreatography (mean difference, 13%; 95% CI, 7.9 to 18; p < 0.001) (Table 2).
Receipt of systemic therapy and time to radiotherapy and systemic therapy after initial diagnosis did not differ significantly between the occult cases and matched group (Table 3). Occult cases compared to matched patients were less likely to have surgical resections (mean difference, 20.2%; 95% CI, 8.5 to 31.8; p=0.001) and receive radiotherapy (mean difference, 15.7%; 95% CI, 6 to 25.3; p=0.005) (Table 3). Among all patients who had surgical resections, the time to surgery was longer for cases compared to matched patients (mean difference, 1 month; 95% CI, 0.17 to 1.85; p=0.01) (Table 3). Among all patients who received systemic therapy, cases were more likely to receive platinum drugs (mean difference, 20.7%; 95% CI, 5.3 to 36.1; p=0.01), anthracyclines (mean difference, 15.7%; 95% CI, 3.4 to 28.1; p=0.002) and taxanes (mean difference, 8.9%; 95% CI, 3 to 18.5; p=0.01) and less likely to receive biological targeted therapy (mean difference, 18%; 95% CI, 8.7 to 27.3; p=0.005) than matched patients (Table 3). Among patients who received biological targeted therapy, cases were more likely to receive bevacizumab (mean difference, 16%; 95% CI, 6.8 to 25.2; p=0.01) compared to the matched group (Table 3). Table 3 shows the treatment characteristics of cases, matched group with obvious GI tumours and all patients with obvious GI tumours (n=1,573).
Cases had worse OS compared to matched patients (2-year OS, 30% vs. 41.3%, p=0.01; median OS, 14.2 months vs. 20.3 months, Fig. 1) (HR, 1.44; 95% CI, 1.1 to 1.9; p=0.01) (Table 4). In a Cox proportional-hazard regression analysis, having an occult compared to an obvious GI tumour became a nonsignificant independent predictor of OS when controlling for use of surgery, radiation therapy and chemotherapy (Table 4). In this analysis, receipt of surgical resection and chemotherapy were significant independent predictors of OS (Table 4). No interactions between treatment and primary tumour status (occult vs. obvious) were identified.
In subgroup analyses, the time from diagnosis to surgery was a significant independent predictor of OS in patients treated with surgery (HR for one month increase in wait time, 1.09; 95% CI, 1.04 to 1.16; p=0.01). The times from diagnosis to receipt of radiation therapy and systemic therapy were not significant independent predictors of OS. Similarly, the type of chemotherapeutic agents received (platinum, taxanes and anthracyclines vs. other chemotherapeutic combinations) was not an independent significant predictor of OS in patients treated with chemotherapy. However, receipt of biological targeted therapy was associated with survival advantage in patients treated with systematic therapy from the case and matched group (HR, 0.5; 95% CI, 0.08 to 0.83; p=0.001).
To our knowledge, the present study is the first to determine the incidence of metastatic cancer of occult GI primary tumours through a population-based analysis. With GI cancers being the most frequent occult primary tumours identified in men and the second most frequent in women with metastatic cancer of uncertain primary, oncologists should maintain a high index of suspicion in GI origins of disease when conducting clinical, surgical, pathological, and radiological evaluations of these patients. It is necessary to understand the natural history of GI cancers because the incidence, prognosis, and recommended treatment of these tumours vary with anatomical location and histological subtype.
The absence of accurate determination of GI primary tumours early in the course of metastatic cancer appears to be associated with fewer diagnostic GI endoscopic examinations during the diagnostic workup, less frequent surgical intervention and use of biological targeted therapy such as bevacizumab, longer time to surgical interventions and greater use of empiric (i.e., broad-spectrum) and more toxic chemotherapeutic drugs such as platinum drugs, taxanes and anthracyclines. Less exposure to surgery and biological targeted therapy and a longer time to receive surgery were all independently associated with higher risk of death and appeared to account for a large portion of the observed 45% increase in risk of mortality for patients with occult GI tumours. This association should be interpreted with caution as it might also be due to unexplained differences in tumour biology, disease burden and/or the functional status of patients differing between cases and the matched group. However, it is still reasonable to hypothesize that many patients were rendered unsuitable for certain effective and targeted cancer treatment and treated with more intensive empiric cytotoxic chemotherapy for their metastatic disease due to the uncertainty of primary tumour site. The implication is that with the growing availability of more effective personalized treatments, it is important to determine GI primary tumour sites early in the course of metastatic disease for timely use of the best systemic and local treatment to optimize patients’ survival and quality of life. The current Canadian clinical practice has not been influenced by the recently emerged gene expression profiling assays to help identify the primary tumour in metastatic cancer [4,25-27]. These techniques complement current traditional diagnostic procedures (e.g., immunohistochemical analyses, endoscopies, CT scans, X-rays, MRI scans, etc.) when dealing with diagnostic difficulties so that the primary tumour can be classified early in the course of metastatic disease [28,29].
A precision medicine approach can often be applied to the treatment of metastatic GI cancers [15]. There are now 10 biological targeted therapies for these cancers that have been approved by the U.S. Food and Drug Administration for clinical use (e.g., cetuximab, panitumumab, and bevacizumab for colorectal cancer, trastuzumab, and regorafenib for gastric and gastroesophageal cancer) and many others are in various phases of development [15]. Generally, these targeted therapies are studied, approved, and reimbursed solely within the context of an identified GI primary tumour location. In addition, selection of these treatments is not only dependent on the biologic characteristics of individual GI tumour (e.g., KRAS mutation and anti-epidermal growth factor receptor status) but also on knowledge of GI primary tumour sites to interpret mutation results. For instance, knowledge of KRAS mutation status has quite different implications depending on whether the primary site is lung versus colon. Therefore, information about the GI primary tumour location and its inherent biologic characteristics are both necessary and complementary for patients to access new personalized treatments of metastatic GI cancers.
Although this is a retrospective cohort study and our results must be considered hypothesis generating, the incidence of occult GI primary tumours and their impact on therapeutic decision making and patient outcomes are unlikely to be studied in prospective designed analyses. This is because metastatic cancer of occult GI source by definition cannot be identified a priori and the size of any prospective investigation would be too large to be feasible and would take several years to complete. Furthermore, the randomization of metastatic patients potentially considered to have occult GI primary tumours to different treatment modalities might not be considered ethical due to the existence of sitespecific therapies and the possibility other therapeutic strategies might be less effective. Our retrospective cohort study is an example of an alternative approach. This study used rigorous linkage of high quality population data from comprehensive heath administrative databases and yielded true incidence rates of occult GI primary tumours. We have taken special care to avoid sources of bias and confounding in our study by conducting a matched cohort analysis where the matched patient group with obvious GI tumours clearly had the same underlying population as the cases with occult GI tumours and were matched on all known patient and tumour characteristics. In fact, the smaller number of patients included in our matched cohort analysis compared to our overall cohort permits future investigation of more detailed and expensive risk factors of having an occult GI tumour. For instance, important factors associated with diagnostic workup obtained from detailed medical histories or biologic markers such as specialist referrals and type and frequency of immunohistochemistry tests (i.e., information not collected by the databases used for this study) become feasible to investigate in order to understand the actual diagnostic barriers in patients with occult GI tumours.
In conclusion, GI tumours are the most common occult primary tumours detected in men and the second most common detected in women presenting with metastatic cancer of uncertain primary. Currently, patients with occult GI primary tumours are potentially being undertreated with available GI site-specific and targeted therapies. It may be beneficial to determine the occult GI primary tumour site early in the course of metastatic cancer to enable more effective therapies and improve survival outcomes.
ACKNOWLEDGMENTS
We thank the Department of Epidemiology and Cancer Registry of CancerCare Manitoba and Manitoba Health, Healthy Living and Seniors for its support throughout the study. The results and conclusions are those of the authors, and no official endorsement by Manitoba Health, Healthy Living and Seniors is intended or should be inferred.
This work was supported by the Canadian Institutes of Health Research (CIHR) (Operating Grant #231890; G.S.Z. [PI]); the CIHR Strategic Training Program in Cancer Research and Technology Transfer (CaRTT) and Academic Development Grant from Western University to M.B.H.; the Canada Research Chairs program to G.S.Z., P.K.R. and S.M.M.; and the Great-West Life, London Life and Canada Life Junior Investigator of the Canadian Cancer Society (Grant #2011-700644) to S.M.M.. Study sponsors had no role in the study design, collection, analysis, and interpretation of the data and in the writing of the report.
References
1. Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian cancer statistics 2016. Toronto, ON: Canadian Cancer Society;2016.
2. Coda S, Thillainayagam AV. State of the art in advanced endoscopic imaging for the detection and evaluation of dysplasia and early cancer of the gastrointestinal tract. Clin Exp Gastroenterol. 2014; 7:133–50.

3. Hainsworth JD, Rubin MS, Spigel DR, Boccia RV, Raby S, Quinn R, et al. Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: a prospective trial of the Sarah Cannon research institute. J Clin Oncol. 2013; 31:217–23.

4. Varadhachary GR, Talantov D, Raber MN, Meng C, Hess KR, Jatkoe T, et al. Molecular profiling of carcinoma of unknown primary and correlation with clinical evaluation. J Clin Oncol. 2008; 26:4442–8.

5. Pentheroudakis G, Greco FA, Pavlidis N. Molecular assignment of tissue of origin in cancer of unknown primary may not predict response to therapy or outcome: a systematic literature review. Cancer Treat Rev. 2009; 35:221–7.

6. von Riedenauer WB, Janjua SA, Kwon DS, Zhang Z, Velanovich V. Immunohistochemical identification of primary peritoneal serous cystadenocarcinoma mimicking advanced colorectal carcinoma: a case report. J Med Case Rep. 2007; 1:150.

7. Laroia ST, Sasturkar S, Rastogi A, Pamecha V. Solitary hypervascular liver metastasis from neuroendocrine tumor mimicking hepatocellular cancer: all that glitters is not gold. Indian J Nucl Med. 2015; 30:42–6.

8. Gunay Y, Demiralay E, Demirag A. Pancreatic metastasis of high-grade papillary serous ovarian carcinoma mimicking primary pancreas cancer: a case report. Case Rep Med. 2012; 2012:943280.

9. Kayilioglu SI, Akyol C, Esen E, Cansiz-Ersoz C, Kocaay AF, Genc V, et al. Gastric metastasis of ectopic breast cancer mimicking axillary metastasis of primary gastric cancer. Case Rep Gastrointest Med. 2014; 2014:232165.
10. Mahmud N, Ford JM, Longacre TA, Parent R, Norton JA. Metastatic lobular breast carcinoma mimicking primary signet ring adenocarcinoma in a patient with a suspected CDH1 mutation. J Clin Oncol. 2015; 33:e19–21.

11. Abid A, Moffa C, Monga DK. Breast cancer metastasis to the GI tract may mimic primary gastric cancer. J Clin Oncol. 2013; 31:e106–7.

12. Ongom PA, Odida M, Lukande RL, Jombwe J, Elobu E. Metastatic colorectal carcinoma mimicking primary ovarian carcinoma presenting as 'giant' ovarian tumors in an individual with probable Lynch syndrome: a case report. J Med Case Rep. 2013; 7:158.

13. Li HC, Schmidt L, Greenson JK, Chang AC, Myers JL. Primary pulmonary adenocarcinoma with intestinal differentiation mimicking metastatic colorectal carcinoma: case report and review of literature. Am J Clin Pathol. 2009; 131:129–33.
14. Benoit MF, Hannigan EV, Smith RP, Smith ER, Byers LJ. Primary gastrointestinal cancers presenting as gynecologic malignancies. Gynecol Oncol. 2004; 95:388–92.

15. Chhatrala R, Thanavala Y, Iyer R. Targeted therapy in gastrointestinal malignancies. J Carcinog. 2014; 13:4.

16. Hannouf MB, Zaric GS. Cost-effectiveness analysis using registry and administrative data. In : Zaric GS, editor. Operations research and health care policy. New York: Springer;2013. p. 341–61.
17. Hannouf MB, Brackstone M, Xie B, Zaric GS. Evaluating the efficacy of current clinical practice of adjuvant chemotherapy in postmenopausal women with early-stage, estrogen or progesterone receptor-positive, one-to-three positive axillary lymph node, breast cancer. Curr Oncol. 2012; 19:e319–28.

18. Hannouf MB, Xie B, Brackstone M, Zaric GS. Cost effectiveness of a 21-gene recurrence score assay versus Canadian clinical practice in post-menopausal women with early-stage estrogen or progesterone-receptor-positive, axillary lymphnode positive breast cancer. Pharmacoeconomics. 2014; 32:135–47.

19. Hannouf MB, Xie B, Brackstone M, Zaric GS. Cost-effectiveness of a 21-gene recurrence score assay versus Canadian clinical practice in women with early-stage estrogen- or progesterone-receptor-positive, axillary lymph-node negative breast cancer. BMC Cancer. 2012; 12:447.

20. Greco FA, Spigel DR, Yardley DA, Erlander MG, Ma XJ, Hainsworth JD. Molecular profiling in unknown primary cancer: accuracy of tissue of origin prediction. Oncologist. 2010; 15:500–6.

21. Hannouf MB, Winquist E, Mahmud SM, Brackstone M, Sarma S, Rodrigues G, et al. Cost-effectiveness of using a gene expression profiling test to aid in identifying the primary tumour in patients with cancer of unknown primary. Pharmacogenomics J. 2017; 17:286–300.

22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40:373–83.

23. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011; 46:399–424.

24. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010; 25:1–21.

25. Bridgewater J, van Laar R, Floore A, Van'T Veer L. Gene expression profiling may improve diagnosis in patients with carcinoma of unknown primary. Br J Cancer. 2008; 98:1425–30.

26. Monzon FA, Koen TJ. Diagnosis of metastatic neoplasms: molecular approaches for identification of tissue of origin. Arch Pathol Lab Med. 2010; 134:216–24.

27. Pillai R, Deeter R, Rigl CT, Nystrom JS, Miller MH, Buturovic L, et al. Validation and reproducibility of a microarray-based gene expression test for tumor identification in formalin-fixed, paraffin-embedded specimens. J Mol Diagn. 2011; 13:48–56.

Fig. 1.
Overall survival analyses comparing patients with occult gastrointestinal (GI) primary tumours to patients with obvious GI primary tumours.
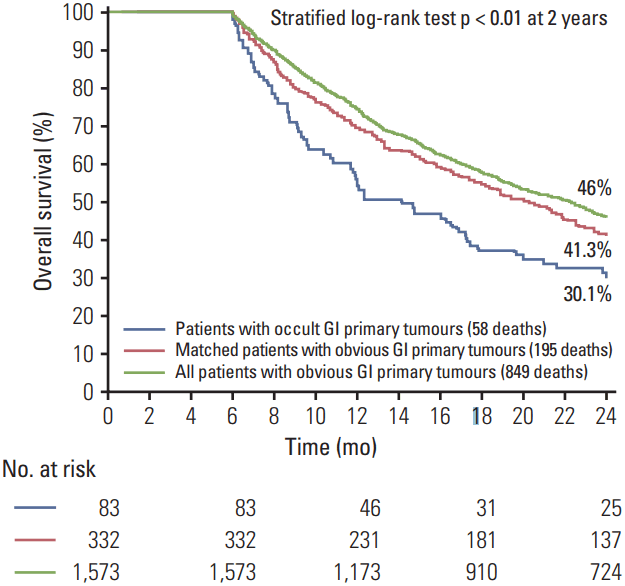
Table 1.
Primary tumour site of 5,953 patients diagnosed with metastatic cancer by sex and diagnostic status of primary tumour
Table 2.
Baseline patient and tumour characteristics of 1,656 patients diagnosed with metastatic GI cancer by diagnostic status of their primary tumours
| Characteristic | Patients with obvious GI primary tumours (n=1,573) | Patients with obvious GI primary tumours (n=83) | p-valuea) | Matched patients with obvious GI primary tumours (n=332)b) | p-valuec) |
|---|---|---|---|---|---|
| Age at initial diagnosis (yr) | |||||
| Mean±SD (range) | 64±12.5 (19-90) | 62±11.7 (36-90) | 0.18 | 62±10.9 (35-90) | 0.90 |
| Year of initial diagnosis | |||||
| 2002-2003 | 269 (17.1) | 14 (16.9) | 0.60 | 57 (17.2) | 0.90 |
| 2004-2005 | 299 (19.0) | 12 (14.5) | 50 (15.06) | ||
| 2006-2007 | 360 (22.9) | 16 (19.3) | 68 (20.5) | ||
| 2008-2009 | 332 (21.1) | 22 (26.5) | 84 (25.3) | ||
| 2010-2011 | 313 (19.9) | 19 (22.9) | 73 (21.98) | ||
| Type of GI diagnostic examination received during the diagnostic workupd) | |||||
| Diagnostic laparoscopy or laparotomy | 104 (6.6) | 10 (12.04) | 0.05 | 32 (9.6) | 0.50 |
| Upper GI endoscopy | 488 (31.02) | 19 (22.9) | 0.10 | 124 (37.3) | 0.01 |
| Lower GI endoscopy | 989 (62.9) | 26 (31.3) | < 0.001 | 151 (45.5) | 0.01 |
| Endoscopic retrograde cholangio-pancreatography | 93 (5.9) | 2 (2.4) | 0.10 | 51 (15.4) | 0.001 |
| Endoscopic ultrasound | 138 (8.8) | 1 (1.2) | 0.01 | 54 (16.3) | < 0.001 |
| Any type of GI diagnostic endoscopic exanimation | 1,286 (81.7) | 37 (44.6) | < 0.001 | 259 (78.01) | < 0.001 |
| Taking of biopsy from a GI site | 609 (38.7) | 17 (20.5) | < 0.001 | 86 (25.9) | 0.30 |
| Abdominal ultrasound | 415 (26.4) | 34 (40.96) | 0.003 | 118 (35.5) | 0.30 |
| CT scan of the abdomen | 1,275 (81.05) | 71 (85.5) | 0.30 | 264 (79.5) | 0.20 |
| MRI scan of the abdomen | 283 (17.99) | 14 (16.9) | 0.80 | 67 (20.2) | 0.50 |
| GI nuclear scan | 2 (0.12) | 0 | > 0.99 | 1 (0.3) | > 0.99 |
| Sex | |||||
| Men | 960 (61.02) | 43 (51.8) | 0.09 | 175 (52.7) | 0.90 |
| Women | 613 (38.97) | 40 (48.2) | 157 (47.3) | ||
| GI primary tumour site | |||||
| Esophagus and gastroesophageal junction | 159 (10.1) | 9 (10.8) | < 0.001 | 38 (11.4) | 0.90 |
| Stomach | 96 (6.1) | 4 (4.8) | 16 (4.8) | ||
| Small intestine | 31 (1.97) | 6 (7.2) | 26 (7.8) | ||
| Colon, rectum, anus, and anal canal | 1,101 (69.99) | 35 (42.2) | 140 (42.2) | ||
| Liver and intrahepatic bile duct | 23 (1.46) | 3 (3.6) | 14 (4.2) | ||
| Gallbladder | 18 (1.1) | 2 (2.4) | 8 (2.4) | ||
| Extrahepatic bile duct | 16 (1.01) | 7 (8.4) | 25 (7.5) | ||
| Pancreas | 127 (8.07) | 15 (18) | 63 (18.9) | ||
| Unspecified GI tract | 2 (0.13) | 2 (2.4) | 2 (0.6) | ||
| Grade differentiation | |||||
| Well differentiated moderately | 65 (4.1) | 6 (7.2) | < 0.001 | 24 (7.2) | 0.90 |
| Moderately differentiated | 826 (52.5) | 18 (21.7) | 73 (21.9) | ||
| Poorly differentiated | 296 (18.8) | 18 (21.7) | 73 (21.9) | ||
| Undifferentiated | 386 (24.6) | 41 (49.4) | 162 (48.8) | ||
| Histology | |||||
| Adenocarcinomas | 1,307 (83.08) | 57 (68.6) | 0.003 | 225 (67.8) | 0.90 |
| Cystic, mucinous and serous | 121 (7.69) | 13 (15.7) | 55 (16.5) | ||
| Squamous, other epithelial, unspecified epithelial, other non-epithelial and undifferentiated | 145 (9.2) | 13 (15.7) | 52 (15.6) | ||
| Time interval between initial cancer diagnosis and identification of primary tumour (mo) | |||||
| Mean±SD (range) | 0.24±0.95 (0-5.9) | 11±4 (6.1-22.9) | < 0.001 | 0.30±1 (0-5.8) | < 0.001 |
| No. of patients (%) | |||||
| ≥ 0 to < 3 | 1,505 (95.7) | 0 | 313 (94.3) | ||
| ≥ 3 to < 6 | 68 (4.3) | 0 | 19 (5.7) | ||
| ≥ 6 to < 9 | 0 | 32 (38.6) | 0 | ||
| ≥ 9 to < 12 | 0 | 21 (25.3) | 0 | ||
| ≥ 12 to < 15 | 0 | 17 (20.5) | 0 | ||
| ≥ 15 to < 24 | 0 | 13 (15.6) | 0 | ||
| No. of metastatic sites | |||||
| 1 | 452 (28.7) | 32 (38.5) | 0.07 | 127 (38.2) | 0.90 |
| 2 | 757 (48.4) | 27 (32.5) | 112 (33.7) | ||
| 3 | 267 (16.9) | 19 (22.8) | 74 (22.2) | ||
| ≥ 4 | 88 (5.6) | 5 (6.02) | 19 (5.7) | ||
| Metastatic sites | |||||
| Digestive system | 1,690 (39.9) | 82 (38.3) | 0.001 | 237 (38.9) | 0.90 |
| Respiratory system | 645 (15.2) | 35 (16.3) | 97 (15.9) | ||
| Female genital system | 52 (1.2) | 14 (6.5) | 39 (6.4) | ||
| Bones and joints | 146 (3.5) | 20 (9.3) | 54 (8.9) | ||
| Lymph nodes | 1,376 (32.5) | 52 (24.3) | 146 (24.0) | ||
| Buccal cavity and pharynx, male genital system, urinary system, brain, endocrine, soft tissue (including heart), skin, hematopoietic and reticuloendothelial systems, others and ill-defined | 326 (7.7) | 11 (5.1) | 36 (5.9) | ||
| With second primary tumour | 60 (3.8) | 0 | 0.07 | 0 | > 0.99 |
| Charlson co-morbidity scoree) | |||||
| Mean±SD (range) | 0.30±0.77 (0-11) | 0.21±0.58 (0-4) | 0.30 | 0.22±0.6 (0-4) | 0.90 |
| Score > 0 | 344 (21.9) | 14 (16.9) | 0.30 | 60 (18) | 0.80 |
| 0 | 1,229 | 69 | 272 | ||
| 1 | 271 | 12 | 48 | ||
| ≥ 2 | 73 | 2 | 12 |
Table 3.
Treatments of 1,656 patients diagnosed with metastatic GI cancer by diagnostic status of their primary tumours
| Characteristic | Patients with obvious GI primary tumours (n=1,573) | Patients with obvious GI primary tumours (n=83) | p-valuea) | Matched patients with obvious GI primary tumours (n=332)b) | p-valueb) |
|---|---|---|---|---|---|
| With surgical resection | 1,055 (67.4) | 30 (36.0) | < 0.001 | 187 (56.3) | 0.001 |
| Time interval between initial cancer diagnosis and surgical resection (mo) | |||||
| Mean±SD (range) | 1.2±2.2 (0-20.1) | 1.9±4.2 (0-20.5) | 0.09 | 0.8±1.6 (0-12.6) | 0.01 |
| ≥ 0 to < 3 | 953 | 25 | 175 | ||
| ≥ 3 to < 6 | 60 | 2 | 8 | ||
| ≥ 6 to < 12 | 29 | 2 | 4 | ||
| ≥ 12 to < 24 | 11 | 1 | 0 | ||
| With radiotherapy | 468 (29.7) | 15 (18.0) | 0.02 | 112 (33.7) | 0.005 |
| Time interval between initial cancer diagnosis and start of radiotherapy (mo) | |||||
| Mean±SD (range) | 6.1±5.6 (0-24) | 6.4±5.1 (0.6-15) | 0.80 | 7.6±6.7 (0.3-24) | 0.50 |
| ≥ 0 to < 3 | 161 | 5 | 33 | ||
| ≥ 3 to < 6 | 147 | 3 | 29 | ||
| ≥ 6 to < 12 | 94 | 5 | 24 | ||
| ≥ 12 to < 24 | 66 | 2 | 26 | ||
| Type of radiotherapy | |||||
| Teletherapy | 346 (73.9) | 11 (73.3) | 0.90 | 87 (70.6) | 0.90 |
| Other types | 122 (26.1) | 4 (26.7) | 33 (29.4) | ||
| With systemic therapy | 1,176 (74.8) | 59 (71.1) | 0.45 | 261 (78.6) | 0.10 |
| Time interval between initial cancer diagnosis and start of systemic therapy (mo) | |||||
| Mean±SD (range) | 3.4±3 (0-23.6) | 3.5±4.5 (0-22.9) | 0.90 | 3.4±3.4 (0-23.6) | 0.90 |
| ≥ 0 to < 3 | 693 | 41 | 162 | ||
| ≥ 3 to < 6 | 359 | 10 | 70 | ||
| ≥ 6 to < 12 | 89 | 4 | 18 | ||
| ≥ 12 to < 24 | 35 | 4 | 11 | ||
| With information about systemic therapy agents received | 876 (74.5) | 46 (77.9) | 0.50 | 200 (76.6) | 0.80 |
| Frequency of systemic therapy agents received | |||||
| Single agents | 130 (14.8) | 10 (21.7) | 0.40 | 55 (27.5) | 0.60 |
| Double agents | 370 (42.3) | 17 (36.9) | 77 (38.5) | ||
| Triple agents or more | 376 (42.9) | 19 (41.3) | 68 (34.0) | ||
| Type of chemotherapeutic agents received | |||||
| Antimetabolitesd) | 841 (96.0) | 44 (95.6) | 0.90 | 190 (95.0) | 0.90 |
| Topoisomerase inhibitorse) | 490 (55.9) | 24 (52.2) | 0.60 | 91 (45.5) | 0.40 |
| Platinum drugsf) | 537 (61.3) | 30 (65.2) | 0.60 | 89 (44.5) | 0.01 |
| Anthracyclinesg) | 57 (6.5) | 10 (21.7) | 0.004 | 12 (6.0) | 0.002 |
| Taxanesh) | 26 (2.9) | 5 (10.8) | 0.01 | 4 (2.0) | 0.01 |
| Others agentsi) | 26 (2.9) | 1 (2.2) | 0.90 | 5 (2.5) | 0.90 |
| With biological targeted therapy | 176 (20.1) | 3 (6.5) | 0.02 | 49 (24.5) | 0.005 |
| Type of biological targeted therapy agents received | |||||
| Bevacizumab | 149 (17.0) | 3 (6.5) | 0.06 | 45 (22.5) | 0.01 |
| Cetuximab | 18 (2.0) | 1 (2.1) | 0.90 | 8 (4.0) | 0.90 |
| Panitumumab | 11(1.3) | 0 | 0.90 | 3 (1.5) | 0.90 |
| Other targeted therapy | 3 (0.3) | 0 | 0.90 | 0 | |
| With support drugs received to control side effects or conditions associated with chemotherapy | 774 (88.4) | 32 (69.6) | < 0.001 | 151 (75.5) | 0.40 |
Table 4.
Adjusted and unadjusted HR for death and 95% CI
| Proportional-hazard modela) |
Unadjusted for treatment characteristic |
Adjusted for receipt of surgical resection, radiation therapy, and chemotherapy |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Occult vs. Obvious |
Occult vs. Obvious |
Receipt of surgical resection (no vs. yes)b) |
Receipt of radiation therapy (no vs. yes)b) |
Receipt of chemotherapy (no vs. yes)b) |
|||||||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| For cases and matched patients with obvious GI tumours (4:1 matching, n=415) | 1.45 | 1.1-1.94 | 0.01 | 1.23 | 0.92-1.66 | 0.16 | 3.88 | 2.97-5.1 | < 0.001 | 0.94 | 0.76-1.27 | 0.50 | 1.39 | 1.15-1.74 | 0.001 |
| For cases and all patients with obvious GI tumours (n=1,656) | |||||||||||||||
| Unadjusted for patient and disease characteristics | 1.68 | 1.3-2.2 | < 0.001 | 1.32 | 1.05-1.7 | 0.04 | 3.3 | 2.8-3.7 | < 0.001 | 1.02 | 0.8-1.17 | 0.60 | 1.60 | 1.38-1.85 | < 0.001 |
| Adjusted for patient and disease characteristicsc) | 1.54 | 1.17-2.05 | 0.002 | 1.31 | 0.99-1.73 | 0.05 | 2.7 | 2.3-3.1 | < 0.001 | 0.95 | 0.73-1.18 | 0.60 | 1.38 | 1.16-1.65 | < 0.001 |
| Adjusted for the estimated propensity score | 1.38 | 1.05-1.8 | 0.02 | 1.19 | 0.9-1.57 | 0.20 | 3.18 | 2.78-3.64 | < 0.001 | 0.99 | 0.78-1.29 | 0.60 | 1.58 | 1.37-1.84 | < 0.001 |
| Adjusted for patient and disease characteristicsc) using inverse probability weighting | 1.73 | 1.44-2.08 | < 0.001 | 1.21 | 0.96-1.46 | 0.06 | 3.31 | 2.91-3.77 | < 0.001 | 0.93 | 0.82-1.05 | 0.09 | 1.48 | 1.28-1.7 | < 0.001 |
a) We created time dependent covariates by creating interactions of the covariates and a function of survival time in the models to test proportionality. When time dependent covariates were not significant then covariates were considered proportional. The proportionality assumption was appropriate for all,




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download