Abstract
Purpose
Materials and Methods
Results
Notes
XW, JSK, and MO are employees of Eli Lilly and Company. MO owns shares in Eli Lilly Pty Ltd. JH Kang is an Advisory Board member for Eli Lilly and Company. MJA has consulted/advised for Eli Lilly and Company. DWK has consulted/advised for Eli Lilly and Company. KP has consulted/advised for Astellas, Astra-Zeneca, AVEO, and Boehringer. EKC, JH Kim, and SWS have no conflicts of interest to disclose.
This study was sponsored by Eli Lilly and Company, manufacturer/licensee of pemetrexed (Alimta®). Medical writing assistance was provided by Rose Boutros, PhD, and Julie Ely, PhD, CMPP, of ProScribe - Envision Pharma Group, and was funded by Eli Lilly. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP2).
References
Fig. 1.
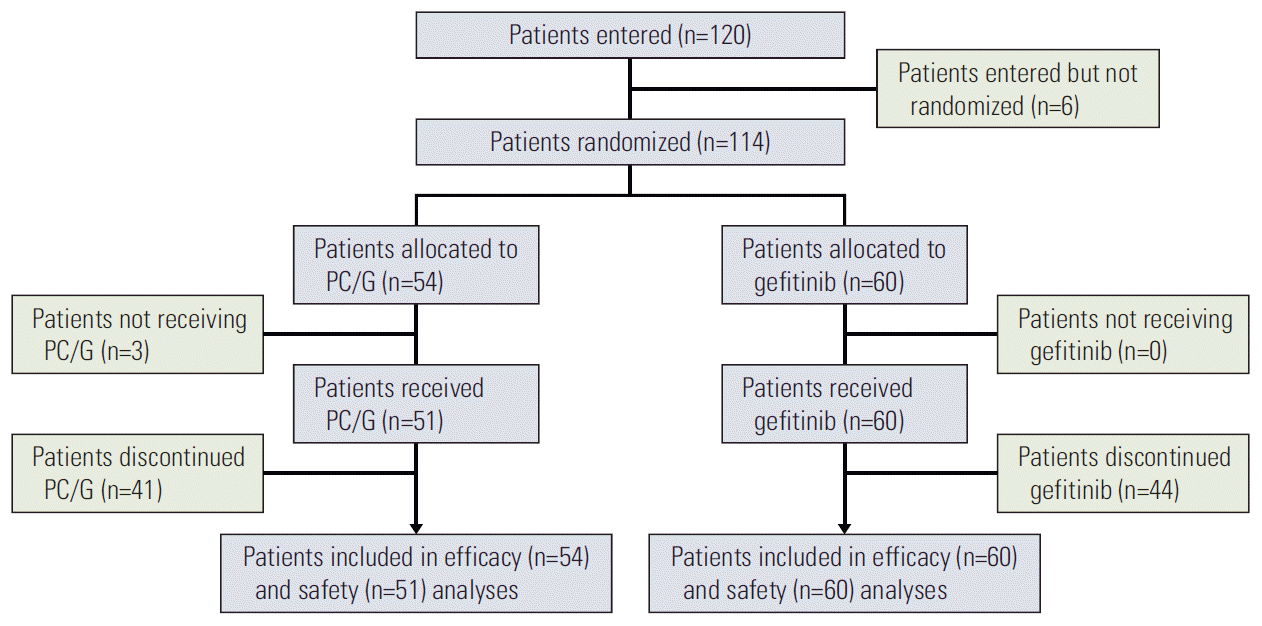
Fig. 2.
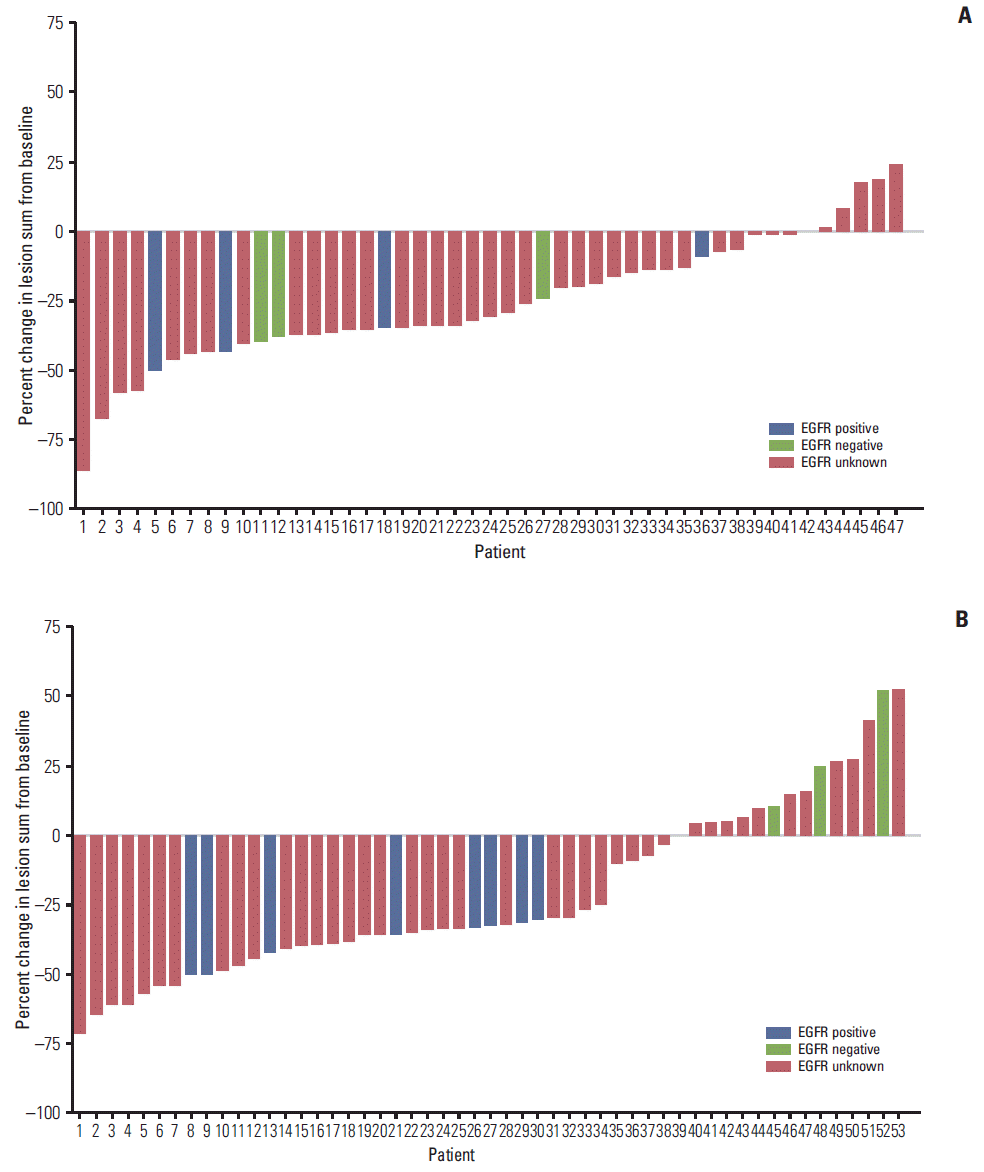
Fig. 3.
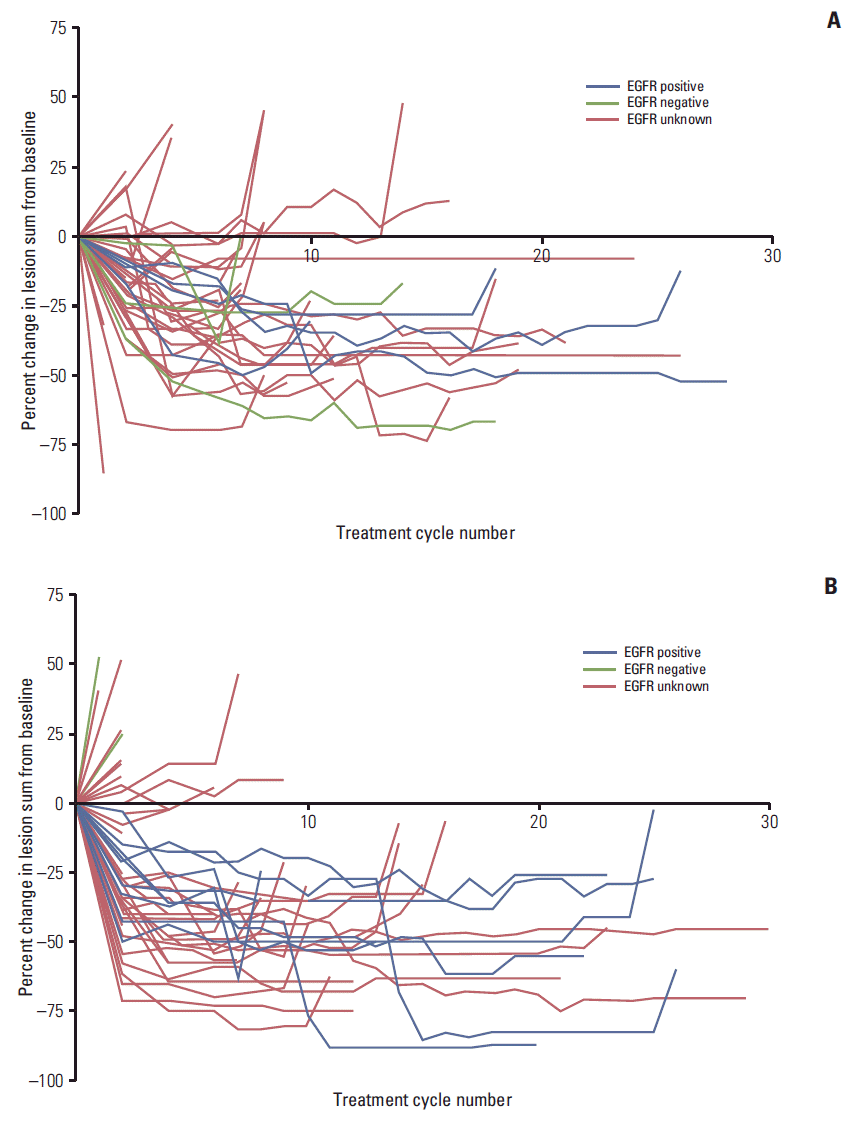
Table 1.
Table 2.
|
Pemetrexed plus cisplatin (n=51) |
Gefitinib (n=60) |
|||
|---|---|---|---|---|
| Grades 1-2 | Grades 3-4 | Grades 1-2 | Grades 3-4 | |
| Patients with ≥ 1 TEAE | 30 (58.8) | 13 (25.5) | 41 (68.3) | 7 (11.7) |
| Hematologic | ||||
| Neutrophils/granulocytes | 5 (9.8) | 9 (17.6) | 0 | 0 |
| Non-hematologic | ||||
| ALT | 0 | 1 (2.0) | 4 (6.7) | 3 (5.0) |
| AST | 0 | 0 | 5 (8.3) | 2 (3.3) |
| Anorexia | 15 (29.4) | 1 (2.0) | 3 (5.0) | 0 |
| Constipation | 4 (7.8) | 0 | 0 | 0 |
| Diarrhoea | 6 (11.8) | 0 | 12 (20.0) | 2 (3.3) |
| Dry skin | 0 | 0 | 8 (13.3) | 0 |
| Fatiguea) | 3 (5.9) | 1 (2.0) | 6 (10.0) | 0 |
| Hair loss | 5 (9.8) | 0 | 2 (3.3) | 0 |
| Hemorrhagea) | 3 (5.9) | 0 | 2 (3.3) | 0 |
| Mucositisa) | 1 (2.0) | 0 | 6 (10.0) | 0 |
| Nausea | 24 (47.1) | 2 (3.9) | 4 (6.7) | 0 |
| Neuropathy, sensory | 9 (17.6) | 0 | 2 (3.3) | 0 |
| Paina) | 6 (11.8) | 0 | 2 (3.3) | 1 (1.7) |
| Pruritus, itching | 5 (9.8) | 0 | 18 (30.0) | 0 |
| Skin rasha) | 3 (5.9) | 0 | 31 (51.7) | 1 (1.7) |
| Vomiting | 11 (21.6) | 3 (5.9) | 0 | 0 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download