Abstract
Purpose
Materials and Methods
Results
Acknowledgments
References
Fig. 1
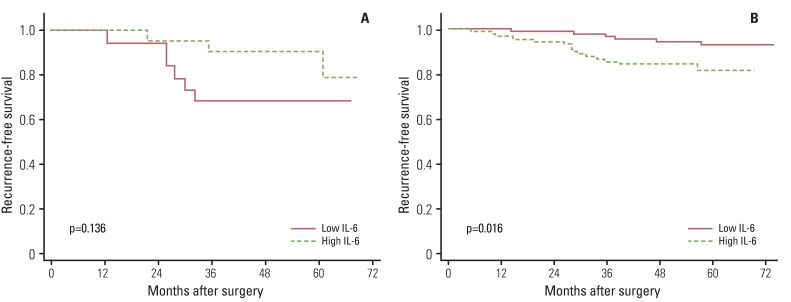
Fig. 2
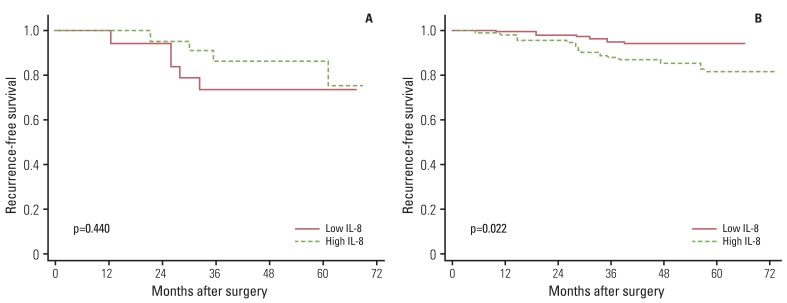
Fig. 3
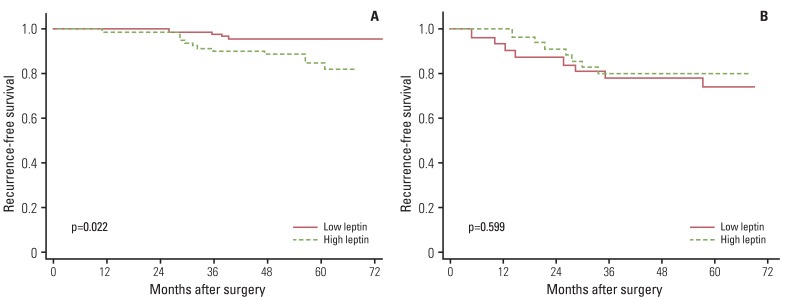
Table 1
|
IL-1β |
IL-6 |
IL-8 |
MCP-1 |
Leptin |
Adiponectin |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | Low | High | Low | High | Low | High | |
| Recurrence | 13 (11.0) | 18 (14.8) | 12 (9.9) | 19 (16.0) | 11 (9.7) | 20 (16.7) | 14 (11.6) | 17 (14.3) | 12 (9.9) | 19 (16.0) | 17 (14.2) | 14 (11.7) |
| Cancer stage | ||||||||||||
| I | 46 (39.0) | 50 (41.0) | 58 (47.9)* | 38 (31.9)* | 54 (45.0) | 42 (35.0) | 51 (42.2)* | 45 (37.8)* | 52 (43.0) | 44 (37.0) | 44 (36.7) | 52 (43.3) |
| II | 47 (39.8) | 51 (41.8) | 44 (36.4)* | 54 (45.4)* | 45 (37.5) | 53 (44.2) | 55 (45.5)* | 43 (36.1)* | 50 (41.3) | 48 (40.3) | 47 (39.2) | 51 (42.5) |
| III | 25 (21.2) | 21 (17.2) | 19 (15.7)* | 27 (22.7)* | 21 (17.5) | 25 (20.8) | 15 (12.4)* | 31 (26.1)* | 19 (15.7) | 27 (22.7) | 29 (24.2) | 17 (14.2) |
| Tumor subtypeb) | ||||||||||||
| Luminal A | 33 (31.1)* | 57 (49.6)* | 47 (42.3) | 43 (39.1) | 42 (38.5) | 48 (42.9) | 49 (43.0) | 41 (38.3) | 52 (47.3) | 38 (34.2) | 46 (41.1) | 44 (40.4) |
| Luminal B | 33 (31.1)* | 29 (25.2)* | 33 (29.7) | 29 (26.4) | 32 (29.4) | 30 (26.8) | 35 (30.7) | 27 (25.2) | 26 (23.6) | 36 (32.4) | 32 (28.6) | 30 (27.5) |
| HER2+ only | 13 (12.3)* | 9 (7.8)* | 11 (9.9) | 11 (10.0) | 10 (9.2) | 12 (10.7) | 6 (5.3) | 16 (15.0) | 7 (6.4) | 15 (13.5) | 10 (8.9) | 12 (11.0) |
| Triple negative | 27 (25.5)* | 20 (17.4)* | 20 (18.0) | 27 (24.6) | 25 (22.9) | 22 (19.6) | 24 (21.1) | 23 (21.5) | 25 (22.7) | 22 (19.8) | 24 (21.4) | 23 (21.1) |
| ER/PR status | ||||||||||||
| ER+/PR+ | 78 (66.1) | 93 (76.2) | 90 (74.4) | 81 (68.1) | 85 (70.8) | 86 (71.7) | 91 (75.2) | 80 (67.2) | 89 (73.6) | 82 (68.9) | 86 (71.7) | 85 (70.3) |
| ER–/PR– | 40 (33.9) | 29 (23.8) | 31 (25.6) | 38 (31.9) | 35 (29.2) | 34 (28.3) | 30 (24.8) | 39 (32.8) | 32 (26.5) | 37 (31.1) | 34 (28.3) | 35 (29.2) |
| HER2 status | ||||||||||||
| HER2+ | 23 (20.7) | 18 (15.5) | 19 (16.5) | 22 (19.6) | 19 (16.7) | 22 (19.5) | 14 (12.1)* | 27 (24.3)* | 14 (12.1)* | 27 (24.3)* | 18 (15.9) | 23 (20.2) |
| HER2– | 88 (79.3) | 98 (84.5) | 96 (83.5) | 90 (80.4) | 95 (83.3) | 91 (80.5) | 102 (87.9)* | 84 (75.7)* | 102 (87.9)* | 84 (75.7)* | 95 (84.1) | 91 (79.8) |
| Tumor size (cm) | ||||||||||||
| <2 | 111 (94.1) | 114 (94.2) | 116 (95.9) | 109 (92.4) | 113 (95.0) | 112 (93.3) | 114 (94.2) | 111 (94.1) | 114 (95.0) | 111 (93.3) | 109 (90.8)* | 116 (97.5)* |
| ≥2 | 7 (5.9) | 7 (5.8) | 5 (4.1) | 9 (7.6) | 6 (5.0) | 8 (6.7) | 7 (5.8) | 7 (5.9) | 6 (5.0) | 8 (6.7) | 11 (9.2)* | 3 (2.5)* |
| Lymph node metastasis | ||||||||||||
| Negative | 66 (55.9) | 70 (57.4) | 79 (65.3)* | 57 (47.9)* | 74 (61.7) | 62 (51.7) | 73 (60.3) | 63 (52.9) | 69 (57.0) | 67 (56.3) | 61 (50.8) | 75 (62.5) |
| Positive | 52 (44.1) | 52 (42.6) | 42 (34.7)* | 62 (52.1)* | 46 (38.3) | 58 (48.3) | 48 (39.7) | 56 (47.1) | 52 (43.0) | 52 (43.7) | 59 (49.2) | 45 (37.5) |
| Ki-67 index (%) | ||||||||||||
| ≥0 and <15 | 47 (43.9) | 66 (55.9) | 60 (52.6) | 53 (47.8) | 58 (51.8) | 55 (48.7) | 58 (49.6) | 55 (50.9) | 61 (55.0) | 52 (45.6) | 55 (48.3) | 58 (52.3) |
| 15-100 | 60 (56.1) | 52 (44.1) | 54 (47.4) | 58 (52.3) | 54 (48.2) | 58 (51.3) | 59 (50.4) | 53 (49.1) | 50 (45.1) | 62 (54.4) | 59 (51.8) | 53 (47.8) |
| Histologic gradec) | ||||||||||||
| G1 | 4 (3.4) | 7 (5.7) | 7 (5.8) | 4 (3.4) | 9 (7.5) | 2 (1.7) | 7 (5.8) | 4 (3.4) | 9 (7.4) | 2 (1.7) | 3 (2.5) | 8 (6.7) |
| G2 | 71 (60.2) | 83 (68.0) | 83 (68.6) | 71 (60.0) | 67 (55.8) | 87 (72.5) | 80 (66.1) | 74 (62.2) | 76 (62.8) | 78 (65.6) | 81 (67.5) | 73 (60.8) |
| G3 | 43 (36.4) | 32 (26.2) | 31 (25.6) | 44 (37.0) | 44 (36.7) | 31 (25.8) | 34 (28.1) | 41 (34.5) | 36 (29.8) | 39 (32.8) | 36 (30.0) | 39 (32.5) |
IL, interleukin; MCP-1, monocyte chemoattractant protein-1; ER, estrogen receptor; PR, progesterone receptor. a)Data are presented as number (%), b)Luminal A (ER+ and/or PR+, HER2- and Ki-67 index<15%), luminal B ([ER+ and/or PR+, HER-, and Ki-67 index≥15%] or [ER+ and/or PR+, and HER2+]), HER2 only (ER-, PR-, and HER2+), triple-negative (ER-, PR-, and HER2-), c)The tumor grade was determined according to the Scarff-Bloom-Richardson classification modified by Elston and Ellis. *p<0.05.
Table 2
|
Patients without recurrence (n=209) |
Patients with recurrence (n=31) |
Rate (%)a) | p-valueb) | |
|---|---|---|---|---|
| Age (yr) | ||||
| <50 | 133 | 18 | 88.1 | 0.511 |
| ≥50 | 76 | 13 | 85.4 | |
| Body mass index (kg/m2) | ||||
| <23 | 113 | 12 | 90.4 | 0.108 |
| ≥23 | 96 | 19 | 83.5 | |
| Smoking status | ||||
| Never | 192 | 28 | 87.3 | 0.768 |
| Ever | 17 | 3 | 85.0 | |
| Alcohol intake | ||||
| Never | 106 | 18 | 85.5 | 0.466 |
| Ever | 103 | 13 | 88.8 | |
| Menopause | ||||
| Premenopause | 122 | 19 | 86.5 | 0.784 |
| Postmenopause | 87 | 12 | 87.9 | |
| Cancer stage | ||||
| I | 88 | 8 | 91.7 | 0.005 |
| II | 88 | 10 | 89.8 | |
| III | 33 | 13 | 71.7 | |
| T stage | ||||
| T1 | 113 | 9 | 92.6 | 0.002 |
| T2 | 88 | 15 | 85.4 | |
| T3 | 7 | 5 | 58.3 | |
| T4 | 1 | 1 | 50.0 | |
| N stage | ||||
| N0 | 123 | 13 | 90.4 | 0.002 |
| N1 | 55 | 8 | 87.3 | |
| N2 | 26 | 5 | 83.9 | |
| N3 | 5 | 5 | 50.0 | |
| Ki-67 index (%) | ||||
| ≥0 and <15 | 105 | 8 | 92.9 | 0.013 |
| 15-100 | 92 | 20 | 82.1 | |
| Histologic gradec) | ||||
| G1 | 10 | 1 | 90.9 | 0.547 |
| G2 | 136 | 18 | 88.3 | |
| G3 | 63 | 12 | 84.0 | |
| Tumor subtypesd) | ||||
| Luminal A | 84 | 9 | 93.3 | 0.034 |
| Luminal B | 53 | 6 | 85.5 | |
| HER2+ only | 17 | 5 | 77.3 | |
| Triple negative | 37 | 10 | 78.7 | |
| ER/PR status | ||||
| ER+/PR+ | 155 | 16 | 90.6 | 0.005 |
| ER–/PR– | 54 | 15 | 78.3 | |
| HER2+ status | ||||
| HER2+ | 32 | 9 | 78.1 | 0.074 |
| HER2– | 164 | 22 | 88.2 | |
| Tamoxifen usee) | ||||
| Yes | 118 | 9 | 92.1 | 0.055 |
| No | 37 | 7 | 84.1 | |
| Anti-HER2 therapyf) | ||||
| Yes | 21 | 3 | 87.5 | 0.825 |
| No | 11 | 6 | 64.7 |
ER, estrogen receptor; PR, progesterone receptor. a)Recurrence-free survival rate, b)Kaplan-Meier statistical method, compared using the log-rank test, c)The tumor grade was determined according to the Scarff-Bloom-Richardson classification modified by Elston and Ellis, d)Luminal A (ER+ and/or PR+, HER2-, and Ki-67 index<15%), luminal B ([ER+ and/or PR+, HER-, and Ki-67 index≥15%] or [ER+ and/or PR+, and HER2+]), HER2 only (ER-, PR-, and HER2+), triple-negative (ER-, PR-, and HER2-), e)The effect of tamoxifen on breast cancer recurrence was compared among patients with hormone receptor positive breast cancer, f)The effect of anti-HER2 therapy, including trastuzumab (Herceptin) and lapatinb (Tykerb), on breast cancer recurrence was compared among patients with HER2+ breast cancer.
Table 3
| Inflammation-related markers | Patients without recurrence | Patients with recurrence | p-valuea) |
|---|---|---|---|
| IL-6 (pg/mL) | |||
| All | 3.5 (3.1-4.2) | 3.8 (3.3-4.6) | 0.176 |
| Tumor subtypeb) | |||
| Luminal A | 3.5 (3.0-4.1) | 3.6 (3.1-4.1) | 1.000 |
| Luminal B | 3.4 (3.0-4.4) | 3.8 (3.5-3.9) | 0.069 |
| HER2+ only | 3.8 (3.0-4.6) | 3.3 (3.3-3.3) | 0.136 |
| Triple negative | 3.6 (3.1-4.1) | 4.5 (3.8-5.7) | 0.024 |
| ER/PR status | |||
| ER+/PR+ | 3.5 (3.0-4.2) | 3.8 (3.2-4.1) | 0.284 |
| ER–/PR– | 3.6 (3.1-4.5) | 2.8 (3.3-5.1) | 0.351 |
| HER2+ status | |||
| HER2+ | 3.7 (3.1-4.4) | 3.3 (3.3-3.8) | 0.300 |
| HER2– | 3.5 (3.0-4.1) | 3.9 (3.5-5.1) | 0.024 |
| IL-8 (pg/mL) | |||
| All | 15.6 (12.2-23.3) | 23.3 (14.4-30.0) | 0.084 |
| Tumor subtypeb) | |||
| Luminal A | 17.8 (13.3-23.3) | 21.1 (15.6-23.3) | 0.388 |
| Luminal B | 15.6 (12.2-22.2) | 30.0 (26.7-33.3) | 0.066 |
| HER2+ only | 17.8 (12.2-20.0) | 15.6 (13.3-16.7) | 0.136 |
| Triple negative | 14.4 (12.2-22.2) | 23.3 (14.4-25.6) | 0.126 |
| ER/PR status | |||
| ER+/PR+ | 15.6 (12.2-23.3) | 23.9 (15.0-30.6) | 0.109 |
| ER–/PR– | 15.6 (12.2-21.1) | 18.9 (13.3-24.4) | 0.351 |
| HER2+ status | |||
| HER2+ | 17.8 (12.2-21.7) | 15.6 (13.3-18.9) | 0.280 |
| HER2– | 15.6 (12.2-23.3) | 23.3 (15.6-30.0) | 0.016 |
| Leptin (pg/mL) | |||
| All | 4.2 (2.6-7.2) | 5.2 (2.1-9.9) | 0.176 |
| Tumor subtypeb) | |||
| Luminal A | 3.4 (2.2-6.7) | 7.6 (5.1-10.0) | 0.089 |
| Luminal B | 4.9 (3.0-7.7) | 5.4 (4.7-10.3) | 0.721 |
| HER2+ only | 6.5 (3.6-8.5) | 7.4 (1.6-8.7) | 0.619 |
| Triple negative | 4.1 (3.2-6.0) | 3.4 (2.0-5.8) | 0.529 |
| ER/PR status | |||
| ER+/PR+ | 4.1 (2.5-7.4) | 5.6 (3.5-10.1) | 0.034 |
| ER–/PR– | 4.8 (3.2-6.6) | 4.0 (1.9-8.7) | 0.821 |
| HER2+ status | |||
| HER2+ | 5.9 (3.5-7.9) | 8.7 (1.6-10.5) | 0.230 |
| HER2– | 3.7 (2.5-6.5) | 5.1 (2.2-5.9) | 0.174 |
Values are presented as number or median (interquartile range). IL, interleukin; ER, estrogen receptor; PR, progesterone receptor. a)The median test was used for identification of significant differences, b)Luminal A (ER+ and/or PR+, HER2-, and Ki-67 index <15%), luminal B ([ER+ and/or PR+, HER-, and Ki-67 index≥15%] or [ER+ and/or PR+, and HER2+]), HER2 only (ER-, PR-, and HER2+), triple-negative (ER-, PR-, and HER2-).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download