Abstract
Background:
Insulin-like growth factor-1 receptor (IGF1R) is a membrane receptor-type tyrosine kinase that has attracted considerable attention as a potential therapeutic target, although its clinical significance in non-small cell lung cancer (NSCLC) is controversial. This study aimed to clarify the clinical significance of IGF1R expression in human NSCLC.
Methods:
IGF1R protein expression was evaluated using immunohistochemistry in 372 patients with NSCLC who underwent curative surgical resection (146 squamous cell carcinomas [SqCCs] and 226 adenocarcinomas [ADCs]). We then analyzed correlations between expression of IGF1R and clinicopathological and molecular features and prognostic significance.
Results:
Membranous and cytoplasmic IGF1R expression were significantly higher in SqCCs than in ADCs. In patients with SqCC, membranous IGF1R expression was associated with absence of vascular, lymphatic, and perineural invasion; lower stage; and better progression-free survival (PFS) (hazard ratio [HR], 0.586; p = .040). In patients with ADC, IGF1R expression did not have a significant prognostic value; however, in the subgroup of epidermal growth factor receptor (EGFR)-mutant ADC, membranous IGF1R expression was associated with lymphatic and perineural invasion, solid predominant histology, and higher cancer stage and was significantly associated with worse PFS (HR, 2.582; p = .009).
Conclusions:
Lung ADC and SqCC showed distinct IGF1R expression profiles that demonstrated prognostic significance. High membranous IGF1R expression was predictive of poor PFS in EGFR-mutant lung ADC, while it was predictive of better PFS in SqCC. These findings will help improve study design for subsequent investigations and select patients for future anti-IGF1R therapy.
Epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) have been used to treat non-small cell lung cancer (NSCLC), and a better response and prolonged survival have been observed in patients who harbor EGFR mutations [1,2]. However, despite a dramatic response, most NSCLC patients experience drug resistance and tumor progression. Two major resistance mechanisms of a secondary point mutation of T790M and MET gene amplification have been reported [3,4]. However, the resistance mechanism remains largely unknown.
Insulin-like growth factor-1 receptor (IGF1R) is a membrane receptor-type tyrosine kinase that plays a crucial role in cancer cell proliferation, inhibition of apoptosis, angiogenesis, and anchorage-independent growth via the phosphatidylinositol 3-kinase-AKT and RAS/RAF/mitogen activated protein kinase signaling pathways [5,6]. In addition, both in vitro and in vivo studies have revealed extensive crosstalk between EGFR and IGF1R signaling on multiple levels [7-10]. These data indicate that IGF1R can lead to acquired resistance against EGFR-targeted drugs, and targeting both receptors could provide better efficacy in cancer treatment by overcoming drug resistance [11,12].
Despite extensive research to clarify the clinical significance of IGF1R in NSCLC, the characteristics and implicated prognostic value remain controversial. Cappuzzo et al. [10] have suggested that patients who have high IGF1R expression and are receiving gefitinib therapy might have improved outcomes compared with those with lower expression. In addition, Kikuchi et al. [13] reported that low IGF1R expression was associated with poor prognosis in lung adenocarcinomas (ADCs). In contrast, Tsuta et al. [14] revealed that, in surgically treated patients, IGF1R protein expression, copy number and IGF1R bright-field in-situ hybridization positivity did not correlate with overall survival. These discrepancies might partly be attributable to the heterogeneity of the study groups including ethnicity, histologic subtypes, and molecular subtypes. To address these differences, we investigated the expression and clinical significance of IGF1R expression in histologically and genotypically specified subgroups of NSCLC.
We collected tumor samples from 372 patients who underwent curative surgical resection for NSCLC at Seoul National University Bundang Hospital in Korea, between May 2003 and December 2008. Patients who did not undergo curative resection and those who had a history of malignancy, preoperative chemotherapy, or radiotherapy were excluded. Smoking status was defined as never-smoker (<100 lifetime cigarettes) or smoker. Tumors were staged using the American Joint Committee on Cancer (AJCC) TNM classification of malignant tumors seventh edition criteria, and the histological type and grade of differentiation of tumors were determined according to the classification system developed by the World Health Organization fourth edition [15,16]. Overall survival (OS) was measured from the date of lung cancer surgery until the time of death, and progression-free survival (PFS) was measured from the date of surgery until recurrence or death. Clinicopathological characteristics of the patients are summarized in Table 1. All patients provided written informed consent, and this study was approved by the Institutional Review Board (IRB) of Seoul National University Bundang Hospital.
Formalin-fixed paraffin-embedded tissues were sectioned at a thickness of 4 µm and stained with rabbit monoclonal antibody against human IGF1R (G11, Ventana Medical Systems, Tucson, AZ, USA) using an automated immunostainer (Ventana Medical Systems). Placental tissue was used as a positive control, and non-immune serum was used as a negative control instead of the primary antibody. Adjacent normal-appearing bronchial epithelium within each tissue section served as an internal reference.
The H-score (semiquantitative system with a total score range of 0–300) was used to evaluate immmunohistochemical staining, and membranous and cytoplasmic staining were evaluated separately. The percentage of positive cells (0%–100%) was multiplied by the dominant intensity score of staining. The intensity score was defined as follows: 0, no appreciable staining; 1, barely detectable staining; 2, distinct brown staining; and 3, strong dark brown staining. A total score range of 0 to 300 was generated for each sample, where median score was used as a cutoff value. All of the slides were evaluated by two pathologists (E.P. and H.K.) in a blinded manner. If discrepancies occurred, a consensus score was reached.
In non-neoplastic lung tissue, the distribution of IGF1R expression was different among cell types. Bronchial basal cells showed constant weak membranous and distinct cytoplasmic expression of IGF1R. Alveolar pneumocytes and alveolar macrophages showed negative to negligible cytoplasmic positivity. In NSCLC, the prevalence of IGF1R expression was significantly higher in squamous cell carcinoma (SqCC) compared with ADC (p = .000), and representative examples are shown in Fig. 1.
Membranous and cytoplasmic IGF1R expression were evident in the 146 patients with SqCC. Membranous IGF1R expression was identified in 100 of 146 patients (68.5%) and was significantly associated with absence of vascular invasion (p = .039), absence of lymphatic invasion (p = .047), absence of perineural invasion (p = .027), lower stage (p = .011), and better PFS (p = .011) and OS (p = .034) compared to the negative subgroup (Table 2, Fig. 2A, B). Multivariate analysis indicated that membranous IGF1R expression was an independent prognostic factor for better PFS (hazard ratio [HR], 0.586; 95% confidence interval [CI], 0.352 to 0.975; p = .040) but not OS (HR, 0.550; 95% CI, 0.302 to 1.002; p = .051). Cytoplasmic IGF1R expression was identified in 107 of 146 patients (73.3%) and tended to correlate with younger age (p = .021) and absence of pleural invasion (p = .013). Patients were additionally divided into four groups: group I that was positive for both membranous and cytoplasmic expression (M+/C+), group II that was membranous expression-positive and cytoplasmic expression-negative (M+/C–), group III that was membranous expression-negative and cytoplasmic expression-positive (M–/C+), and group IV that was negative for both membranous and cytoplasmic expression (M–/C–). The groups included 85, 16, 24, and 21 patients, respectively. On univariate analysis, group II that was membranous expression-positive and cytoplasmic expression-negative (M+/C–) showed the best PFS (p = .021) and OS (p = .028), followed by group I (M+/C+), group IV (M–/C–), and group III (M–/C+).
In ADC patients, IGF1R expression was predominantly cytoplasmic with weak membranous staining. Positive membranous IGF1R expression was identified in 31 of 226 patients (13.7%) and was significantly associated with perineural invasion (p = .040), although the measure had no significant prognostic value. Cytoplasmic expression was identified in 141 of 226 patients (62.4%) and correlated with smaller tumor size (p = .002) and absence of vascular invasion (p = .046), but these results were not statistically significant for PFS or OS.
EGFR mutation was found in 108 of 226 patients (47.8%). The most common mutation was in-frame deletion in exon 19 (57 of 108, 52.8%), followed by missense mutation (L858R) in exon 21 (35 of 108, 32.4%). When ADC samples were stratified into EGFR-mutant and EGFR-wild type subgroups, membranous IGF1R expression was identified in 13 of 108 patients (12.0%) in the EGFR-mutant ADC subgroup. Membranous IGF1R expression was associated with lymphatic invasion (p = .039), perineural invasion (p = .004), solid predominant histology (p = .037), and higher cancer stage (p = .030) and showed a significantly worse PFS (p = .002) and OS (p = .040) compared with those without membranous expression (Table 3, Fig. 2C, D). Multivariate analysis revealed that membranous IGF1R expression was an independent prognostic factor for worse PFS (HR, 2.582; 95% CI, 1.265 to 5.271; p = .009) but not for OS (HR, 1.369; 95% CI, 0.558 to 3.359; p = .492) in patients with EGFR-mutant ADC. Cytoplasmic IGF1R expression was not associated with any clinicopathological parameters in PFS or OS.
In EGFR-wild type ADC, membranous IGF1R expression was identified in 23 of 118 patients (19.5%) and was not correlated with clinicopathological parameters. In contrast, cytoplasmic IGF1R expression was observed in 86 patients (73.5%) who showed significantly better PFS (p = .033) and OS (p = .05) compared to the negative subgroup. No positive membranous IGF1R expression was observed for TKI-treated EGFR-mutant ADC (n = 36). Positive IGF1R cytoplasmic expression was seen in 15 of 36 patients (41.7%) but was not associated with PFS or OS. In addition, IGF1R expression was not correlated with ALK, KRAS, ROS1, or MET mutation (p > .05).
We determined the possible clinical significance of IGF1R expression in NSCLC. The main findings of this study are as follows: (1) the prevalence of IGF1R expression was significantly higher in SqCC compared with ADC; (2) in SqCC, membranous IGF1R expression was associated with significantly better PFS compared with those without membranous expression; (3) in ADC, membranous and cytoplasmic IGF1R expression had no significant prognostic value; (4) in the EGFR-mutant ADC subgroup, membranous IGF1R expression was significantly associated with worse PFS.
In SqCC, membranous IGF1R expression was associated with several good prognostic clinicopathological parameters and showed a linear relationship with PFS. Several previous studies have delineated the association between IGF1R expression and better OS, although the biologic mechanism is unclear [9,21]. One possible explanation is that, because IGF1R is expressed in normal bronchial basal cells, the progenitors of SqCC tumor cells, membranous IGF1R expression on tumor cells could be interpreted as an activated and overexpressed form of the physiological function of the receptor [22]. If the tumor cell displays higher IGF1R expression, it is a more mature and well-differentiated cell that still depends on the normal IGF1R pathway and can be expected to become indolent during the course of the disease. An alternative explanation, based on the fact that the degree of receptor expression would reflect the level of its ligands, is that, if the level of the ligand is higher, more receptors would be occupied and internalized for signaling, while low circulating ligand level would result in receptor overexpression as a compensatory mechanism. Therefore, positive membranous IGF1R expression could be interpreted as a level of IGF1 or IGF2 within or lower than the normal range, which could imply that the tumor has low proliferative activity.
Not only was membranous IGF1R expression significantly associated with better survival in SqCC, but the membranous-positive and cytoplasmic-negative subgroup (M+/C–) had the best PFS and OS in SqCC. This result suggests opposing effects of IGF1R protein expression based on its location. This is the first study to evaluate the cellular location of IGF1R expression in NSCLC and provides better understanding of the relevance of pattern of IGF1R staining in NSCLC.
In ADC patients, IGF1R expression did not affect clinical outcome. However, when we classified these patients on the basis of EGFR-mutation status, the EGFR-mutant ADC subgroup showed strong correlations with several negative prognostic clinicopathological parameters and worse survival. Therefore, IGF1R was identified as a poor prognostic factor in EGFR-mutant ADC, which is opposite the result seen in the SqCC group.
This difference prompted speculation that the role of the IGF1R pathway in ADC might differ according to the presence of EGFR mutations. We assume that, in the EGFR-mutant ADC subgroup, the IGF1R pathway would activate downstream targets on the EGFR, which could promote cellular proliferation, inhibition of apoptosis, angiogenesis, and anchorage-independent growth. In NSCLC, experimental studies have demonstrated that IGF1R activation can underlie resistance to EGFR-targeted therapies, and several clinical trials have indicated that coexpression of EGFR and IGF1R correlates with poor survival [8,23]. Furthermore, Yeo et al. [24] recently reported that IGF1R expression was a negative predictive factor for response to EGFR-TKI treatment in EGFR-mutant ADC patients. Although these data indicate that IGF1R leads to acquired resistance against EGFR-targeted drugs, none of the patients in the EGFR-TKI treated group showed membranous IGF1R expression.
In summary, the implication of IGF1R expression in NSCLC differs according to subtype, and the clinical significance of IGF1R expression should be interpreted after considering the histology and genotype of the tumor. High membranous IGF1R expression might be a biomarker of better PFS in SqCC and of poor PFS in EGFR-mutant lung ADC. These findings will serve to improve study design for subsequent investigations into IGF1R and NSCLC and to select patients for future anti-IGF1R therapy.
ACKNOWLEDGMENTS
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2013-059757) and partly by a grant from the Korea Healthcare Tech-nology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI14C1907). The authors thank J. Patrick Barron, Professor Emeritus, Tokyo Medical University and Adjunct Professor, Seoul National University Bundang Hospital for his pro bono editing of this manuscript.
REFERENCES
1. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–57.

2. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011; 29:2866–74.

3. Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008; 105:2070–5.
4. Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007; 316:1039–43.
5. Ward CW, Garrett TP, McKern NM, et al. The three dimensional structure of the type I insulin-like growth factor receptor. Mol Pathol. 2001; 54:125–32.

6. Blakesley VA, Stannard BS, Kalebic T, Helman LJ, LeRoith D. Role of the IGF-I receptor in mutagenesis and tumor promotion. J Endocrinol. 1997; 152:339–44.

7. Bianconi F, Baldelli E, Ludovini V, Crino L, Flacco A, Valigi P. Computational model of EGFR and IGF1R pathways in lung cancer: a systems biology approach for translational oncology. Biotechnol Adv. 2012; 30:142–53.

8. Ludovini V, Bellezza G, Pistola L, et al. High coexpression of both insulin-like growth factor receptor-1 (IGFR-1) and epidermal growth factor receptor (EGFR) is associated with shorter disease-free survival in resected non-small-cell lung cancer patients. Ann Oncol. 2009; 20:842–9.

9. Dziadziuszko R, Merrick DT, Witta SE, et al. Insulin-like growth factor receptor 1 (IGF1R) gene copy number is associated with survival in operable non-small-cell lung cancer: a comparison between IGF1R fluorescent in situ hybridization, protein expression, and mRNA expression. J Clin Oncol. 2010; 28:2174–80.

10. Cappuzzo F, Tallini G, Finocchiaro G, et al. Insulin-like growth factor receptor 1 (IGF1R) expression and survival in surgically resected non-small-cell lung cancer (NSCLC) patients. Ann Oncol. 2010; 21:562–7.

11. van der Veeken J, Oliveira S, Schiffelers RM, Storm G, van Bergen En Henegouwen PM, Roovers RC. Crosstalk between epidermal growth factor receptor- and insulin-like growth factor-1 receptor signaling: implications for cancer therapy. Curr Cancer Drug Targets. 2009; 9:748–60.
12. Morgillo F, Hong WK, Lee H. Insulin-like growth factor-1 receptor/epidermal growth factor receptor (EGFR) heterodimerization and resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2006; 24:13032.
13. Kikuchi R, Sonobe M, Kobayashi M, et al. Expression of IGF1R is associated with tumor differentiation and survival in patients with lung adenocarcinoma. Ann Surg Oncol. 2012; 19 Suppl 3:S412–20.

14. Tsuta K, Mimae T, Nitta H, et al. Insulin-like growth factor-1 receptor protein expression and gene copy number alterations in non-small cell lung carcinomas. Hum Pathol. 2013; 44:975–82.

15. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2010.
16. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart. 4th ed. Lyon: IARC Press;2015.
17. Lee HJ, Xu X, Kim H, et al. Comparison of direct sequencing, PNA clamping-real time polymerase chain reaction, and pyrosequencing methods for the detection of EGFR mutations in non-small cell lung carcinoma and the correlation with clinical responses to EGFR tyrosine kinase inhibitor treatment. Korean J Pathol. 2013; 47:52–60.
18. Kim H, Yoo SB, Choe JY, et al. Detection of ALK gene rearrangement in non-small cell lung cancer: a comparison of fluorescence in situ hybridization and chromogenic in situ hybridization with correlation of ALK protein expression. J Thorac Oncol. 2011; 6:1359–66.
19. Jin Y, Sun PL, Kim H, et al. MET gene copy number gain is an independent poor prognostic marker in Korean stage I lung adenocarcinomas. Ann Surg Oncol. 2014; 21:621–8.
20. Jin Y, Sun PL, Kim H, et al. ROS1 gene rearrangement and copy number gain in non-small cell lung cancer. Virchows Arch. 2015; 466:45–52.
21. Reinmuth N, Kloos S, Warth A, et al. Insulin-like growth factor 1 pathway mutations and protein expression in resected non-small cell lung cancer. Hum Pathol. 2014; 45:1162–8.

23. Gately K, Forde L, Cuffe S, et al. High coexpression of both EGFR and IGF1R correlates with poor patient prognosis in resected non-small-cell lung cancer. Clin Lung Cancer. 2014; 15:58–66.

24. Yeo CD, Park KH, Park CK, et al. Expression of insulin-like growth factor 1 receptor (IGF-1R) predicts poor responses to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in non-small cell lung cancer patients harboring activating EGFR mutations. Lung Cancer. 2015; 87:311–7.
Fig. 1.
Representative immunohistochemistry examples of membranous insulin-like growth factor-1 receptor (IGF1R) expression. IGF1R expression in non-neoplastic lung tissue (A), membranous IGF1R expression in squamous cell carcinoma (B), membranous IGF1R expression of adenocarcinoma (C).
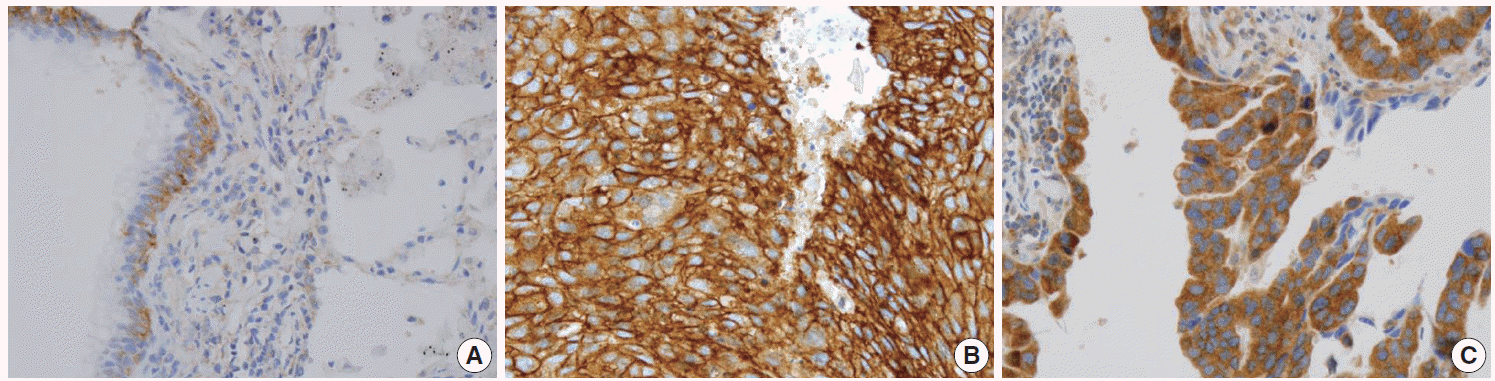
Fig. 2.
Kaplan-Meier survival analysis of progression-free survival and overall survival curves based on membranous insulin-like growth factor-1 receptor (IGF1R) expression. Membranous IGF1R expression had significantly better progression-free survival (A) and overall survival (B) in squamous cell carcinoma. Membranous IGF1R expression in epidermal growth factor receptor (EGFR)-mutant adenocarcinoma had significantly poor progression-free survival (C) and a trend toward poor overall survival (D).
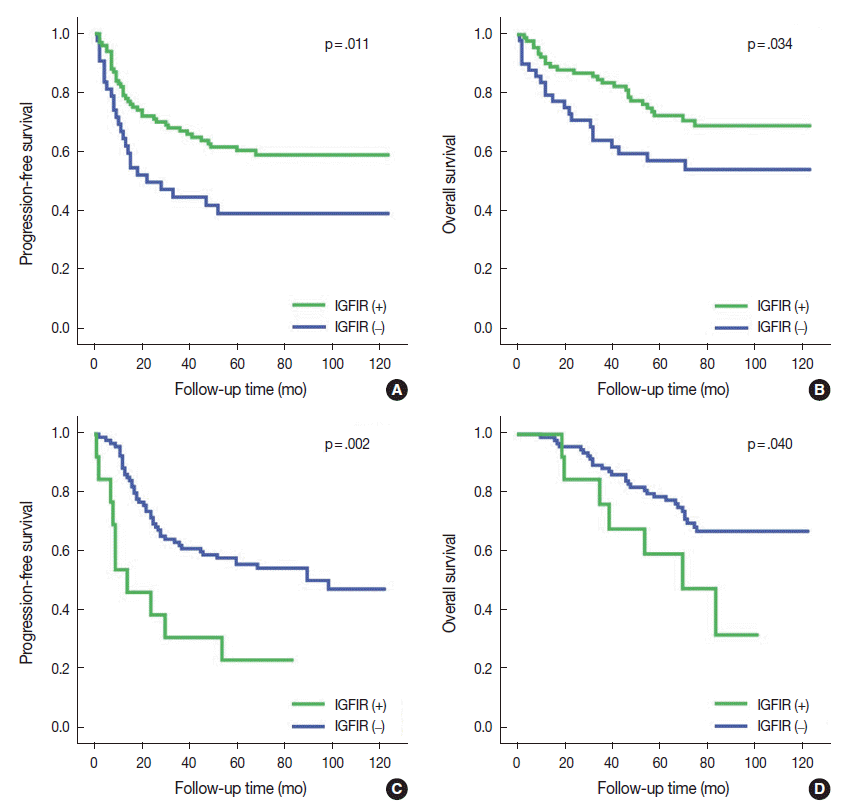
Table 1.
Patient characteristics
Table 2.
Clinicopathological characteristics in relation to expression of IGF1R in SqCC
Table 3.
Clinicopathological characteristics in relation to expression of IGF1R in EGFR-mutant ADC




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download