Abstract
Background
Methods
Results
Notes
REFERENCES
Fig. 1.
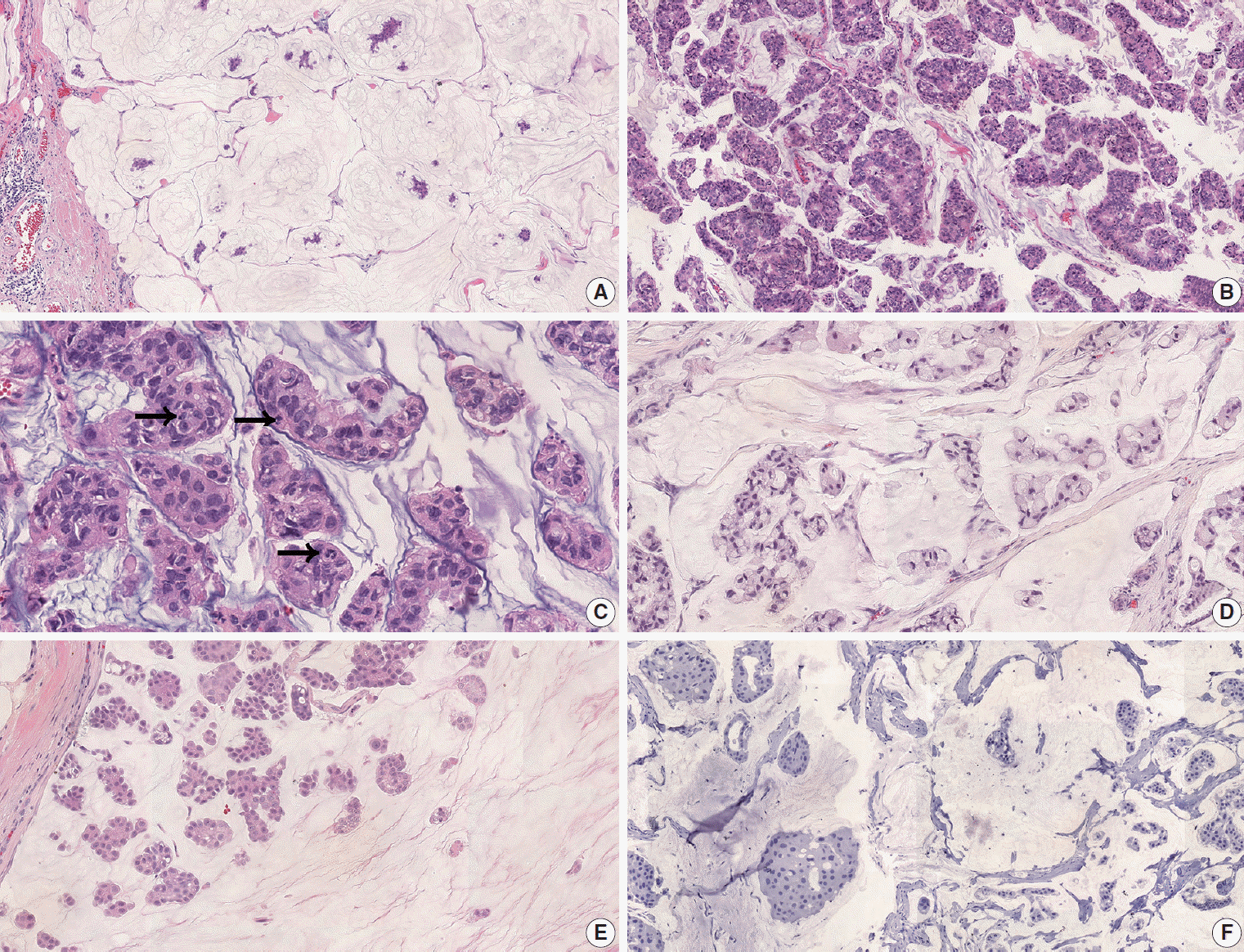
Fig. 2.
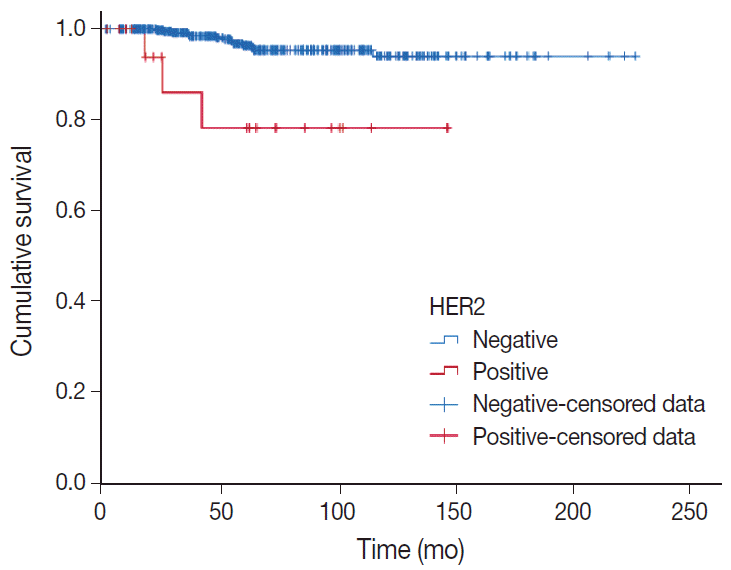
Table 1.
| Case | Age (yr) | Sex | Procedure | Mass size (mm) | T category | No. of positive LNs | N category | Nuclear | Histologic grade | ER | PR | Type A/B | Structure | Recurrence | Follow-up | Trastuzumab | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | F | T | A | 30.0 | 2 | 0 | 0 | 3 | III | - | - | B | SRC | NED | ND | |
| 2 | 41 | F | B | S | 15.0 | 1 | 0 | 0 | 3 | III | + | + | B | None | NED | ND | |
| 3 | 43 | F | T | S | 3.0 | 1 | 0 | 0 | 2 | II | + | + | A | None | NED | ND | |
| 4 | 28 | F | B | S | 15.0 | 1 | 0 | 0 | 3 | III | + | + | B | SRC | NED | ND | |
| 5 | 60 | F | T | A | 70.0 | 3 | 1 | 1 | 3 | III | - | - | A | MP/SRC | NED | ND | |
| 6 | 43 | F | B | S | 4.0 | 1 | 0 | 0 | 2 | II | - | + | A | None | NED | ND | |
| 7 | 53 | F | T | S | 4.5 | 1 | 0 | 0 | 2 | II | + | - | B | SRC | NED | ND | |
| 8 | 46 | F | T | A | 65/60a | 3 | 5 | 2 | 2 | II | - | - | A | MP/SRC | Local recurrence | DOD | ND |
| 9 | 39 | F | T | A | 38.0 | 2 | 0 | 0 | 2 | II | + | + | NA | None | Metastasis to lung and brain | DOC | ND |
| 10 | 55 | F | B | S | 15.0 | 1 | 0 | 0 | 3 | III | + | + | A | MP | NED | NA | |
| 11 | 37 | F | B | S | 55/0a | 3 | NAb | NAb | 2 | II | - | + | B | SRC | NED | Donec | |
| 12 | 46 | F | T | A | 85/70a | 3 | 7 | 2 | 3 | III | + | + | A | None | NED | Donec | |
| 13 | 48 | F | B | A | 90/80a | 3 | 1 | 1 | 2 | II | + | + | A | SRC | NED | Donec | |
| 14 | 63 | F | B | S | 16.0 | 1 | 0 | 0 | 3 | III | + | + | A | SRC | NED | Done | |
| 15 | 40 | F | B | A | 30/10a | 2 | 2 | 1 | NA | NA | + | + | NA | NA | NED | Done | |
| 16 | 38 | F | B | S | 12.0 | 1 | 0 | 0 | 2 | II | - | - | A | MP | NED | Done | |
| 17 | 32 | F | T | A | 16.0 | 1 | 0 | 0 | 3 | III | + | + | A | SRC | NED | Done | |
| 18 | 34 | F | B | S | 40.0 | 2 | 0 | 0 | 2 | II | - | - | A | SRC | NED | Done | |
| 19 | 41 | F | T | A | 41.0 | 2 | 2 | 1 | 3 | III | + | + | B | SRC | NED | Done | |
| 20 | 50 | F | B | S | 16.0 | 1 | 0 | 0 | 2 | II | + | + | A | SRC | NED | Done | |
| 21 | 54 | F | T | A | 16/19a | 1 | 22 | 3 | 3 | III | + | + | A | MP | Metastasis to skin and lung | DOC | Done |
HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; PR, progesterone receptor; F, female; T, total mastectomy; A, axillary lymph node dissection; SRC, signet ring cells; NED, no evidence of disease; ND, not done; B, breast conserving surgery; S, sentinel lymph node biopsy; MP, micropapillary; DOD, died of disease; NA, not applicable; DOC, died of other cause.
Table 2.
| Variable | Total (n = 438) | HER2-negative PMC (n = 417) | HER2-positive PMC (n = 21) | p-value |
|---|---|---|---|---|
| Age (yr) | 49.92 | 50.15 | 45.29 | .073 |
| Sex | ||||
| Female | 431 | 410 | 21 | |
| Male | 7 | 7 | 0 | |
| Tumor size (mm) | 21.3 ± 14.41 | 20.73 ± 13.34 | 32.21 ± 26.55 | < .001 |
| T category | < .001 | |||
| T1 | 242 | 231 (55.5) | 11 (52.4) | |
| T2 | 174 | 169 (40.6) | 5 (23.8) | |
| T3 | 21 | 16 (3.8) | 5 (23.8) | |
| Lymph node statusa | .009 | |||
| Negative | 378 | 365 (88.0) | 13 (65.0) | |
| Positive | 57 | 50 (12.0) | 7 (35.0) | |
| Nuclear gradea | < .001 | |||
| 1 | 200 | 200 (48.0) | 0 | |
| 2 | 217 | 207 (49.6) | 10 (50.0) | |
| 3 | 20 | 10 (2.4) | 10 (50.0) | |
| Histologic gradea | < .001 | |||
| I | 248 | 248 (59.5) | 0 | |
| II | 166 | 156 (37.4) | 10 (50.0) | |
| III | 23 | 13 (3.1) | 10 (50.0) | |
| ER | < .001 | |||
| Negative | 12 | 5 (1.2) | 7 (33.3) | |
| Positive | 424 | 410 (98.8) | 14 (66.7) | |
| PR | .005 | |||
| Negative | 36 | 30 (7.2) | 6 (28.6) | |
| Positive | 400 | 385 (92.8) | 15 (71.4) | |
| KI-67a | .006 | |||
| < 20% | 244 | 236 (84.6) | 8 (53.3) | |
| ≥ 20% | 50 | 43 (15.4) | 7 (46.7) | |
| Type A/Ba | .940 | |||
| A | 301 | 288 (69.2) | 13 (68.4) | |
| B | 134 | 128 (30.8) | 6 (31.6) | |
| EICa | .011 | |||
| Negative | 328 | 318 (78.1) | 10 (50.0) | |
| Positive | 99 | 89 (21.9) | 10 (50.0) | |
| LVIa | .158 | |||
| Absent | 346 | 331 (87.8) | 15 (75.0) | |
| Present | 51 | 46 (12.2) | 5 (25.0) | |
| MPa | .012 | |||
| Absent | 207 | 192 (46.2) | 15 (75.0) | |
| Present | 229 | 224 (53.8) | 5 (25.0) | |
| SRCa | < .001 | |||
| Absent | 376 | 368 (88.5) | 8 (40.0) | |
| Present | 60 | 48 (11.5) | 12 (60.0) | |
| Operation | .182 | |||
| Excision | 7 | 7 (1.7) | 0 | |
| Conserving surgery | 299 | 288 (69.1) | 11 (52.4) | |
| Total mastectomy | 132 | 122 (29.3) | 10 (47.6) | |
| Lymph node dissection | .011 | |||
| Sentinel biopsy | 329 | 318 (76.8) | 11 (52.4) | |
| Axillary dissection | 106 | 96 (23.2) | 10 (47.6) | |
| Chemotherapya | < .001 | |||
| No | 320 | 315 (76.1) | 5 (25.0) | |
| Yes | 114 | 99 (23.9) | 15 (75.0) | |
| Trastuzumab treatmenta | ||||
| No | 9 (45.0) | |||
| Yes | 11 (55.0) | |||
| Radiotherapya | .199 | |||
| No | 119 | 111 (26.9) | 8 (40.0) | |
| Yes | 314 | 302 (73.1) | 12 (60.0) | |
| Hormone therapya | .001 | |||
| No | 11 | 7 (1.7) | 4 (20.0) | |
| Yes | 420 | 404 (98.3) | 16 (80.0) |
Values are presented as number (%) or mean ± standard deviation.
HER2, human epidermal growth factor receptor 2; PMC, pure mucinous carcinoma; ER, estrogen receptor; PR, progesterone receptor; EIC, extensive intraductal component; LVI, lymphovascular invasion; MP, micropapillary pattern; SRC, signet ring cell pattern.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download