Abstract
Background
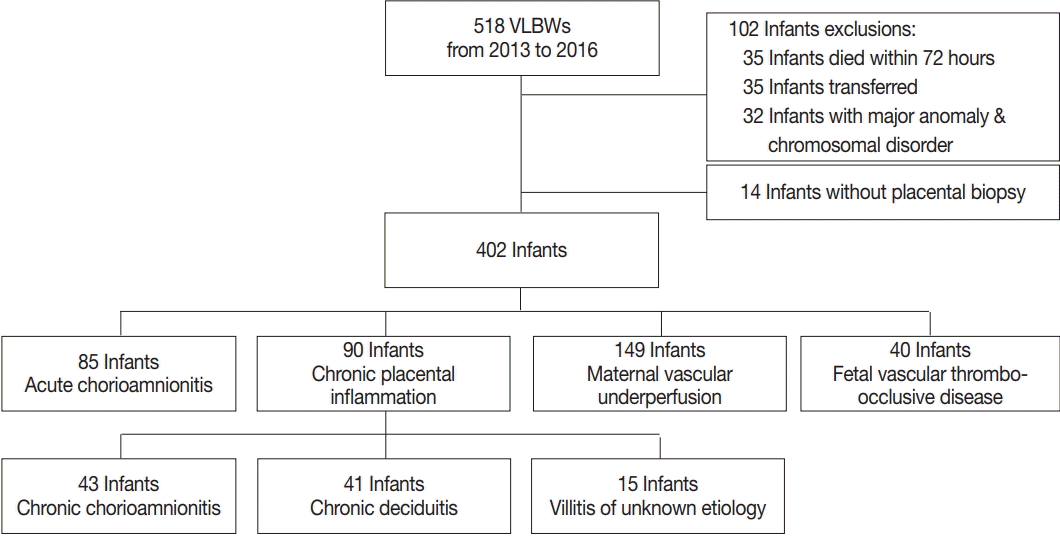
Methods
Results
REFERENCES
Fig. 2.
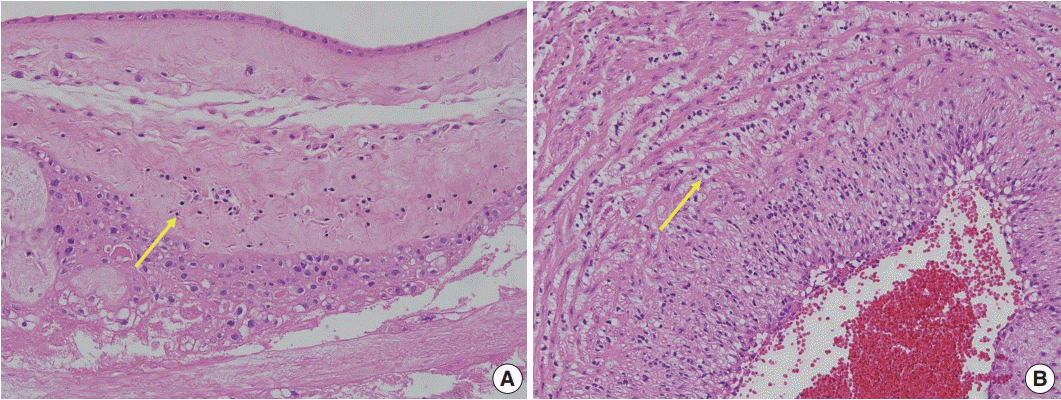
Table 1.
Values are presented as mean ± SD, number (%), or mean (IQR).
IUGR, intrauterine growth retardation; RDS, respiratory distress syndrome; PDA, patent ductus arteriosus; BPD, bronchopulmonary dysplasia; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia; NEC, necrotizing enterocolitis; ROP, retinopathy of prematurity; VEGF, vascular endothelial growth factor; LOS, late-onset sepsis; SD, standard deviation; IQR, interquartile range.
Table 2.
| Variable | Non-CPI (n = 312) | CPI (n = 90) | p-value |
|---|---|---|---|
| Gestational age (wk) | 29.1 ± 2.9 | 28.4 ± 3.2 | .007* |
| Birth weight (g) | 1,064.7 ± 286.9 | 1,012.5 ± 309.1 | .589 |
| Sex | .506 | ||
| Male | 154 (49.3) | 48 (53.3) | |
| Female | 158 (50.6) | 42 (46.7) | |
| Pre-eclampsia | 78 (25.0) | 9 (10.0) | .031* |
| Antenatal steroid use | 284 (91.0) | 79 (87.8) | .398 |
| IUGR | 107 (34.3) | 26 (28.9) | .338 |
| RDS | 177 (56.7) | 61 (67.8) | .068 |
| Significant PDA | 127 (40.7) | 40 (44.4) | .531 |
| Moderate to severe BPD | 79 (25.3) | 26 (28.9) | .497 |
| Postnatal steroid therapy | 72 (23.1) | 24 (26.7) | .496 |
| Duration of oxygen supply (day) | 41.4 ± 38.2 | 41.0 ± 38.1 | .163 |
| Severe neurologic injury (IVH ≥ grade 3 or PVL) | 34 (10.9) | 9 (10) | .051 |
| NEC (≥ stage 2) | 15 (4.8) | 4 (4.4) | .882 |
| LOS | 65 (20.8) | 23 (25.6) | .164 |
| ROP (any stage) | 71 (22.8) | 26 (28.9) | .021* |
| Type 1 ROP | 27 (8.6) | 19 (21.1) | .003* |
| Hospital days (day) | 66.5 ± 36.0 | 72.6 ± 36.1 | .154 |
Values are presented as mean ± SD or number (%).
CPI, chronic placental inflammation; IUGR, intrauterine growth retardation; RDS, respiratory distress syndrome; PDA, patent ductus arteriosus; BPD, bronchopulmonary dysplasia; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia; NEC, necrotizing enterocolitis; LOS, late-onset sepsis; ROP, retinopathy of prematurity; SD, standard deviation.
Table 3.
| Variable |
Any stage ROP |
Type 1 ROP |
||||||
|---|---|---|---|---|---|---|---|---|
| No ROP (n = 305) | ROP (n = 97) | OR (95% CI) | p-value | No type 1 ROP (n = 356) | Type 1 ROP (n = 46) | OR (95% CI) | p-value | |
| Gestational age (wk) | 30.0 ± 2.5 | 25.7 ± 1.7 | 0.872 (0.847–0.897) | < .001* | 30.4 ± 2.8 | 25.1 ± 1.5 | 0.882 (0.853–0.911) | < .001* |
| Birth weight (g) | 1,152.5 ± 239.5 | 740.6 ± 212.9 | 0.993 (0.992–0.995) | < .001* | 1100 ± 267.7 | 680.8 ± 198.7 | 0.993 (0.991–0.995) | < .001* |
| Male | 157 (51.5) | 49 (50.5) | 1.000 (0.329–1.126) | .339 | 179 (50.3) | 23 (50.0) | 1.000 (0.504–1.305) | .219 |
| Pre-eclampsia | 62 (20.3) | 7 (7.2) | 0.919 (0.383–2.205) | .851 | 66 (18.5) | 3 (6.5) | 0.295 (0.089–0.981) | .046* |
| Antenatal steroid | 271 (88.9) | 92 (94.8) | 1.547 (0.484–4.942) | .461 | 321 (90.2) | 42 (91.3) | 1.145 (0.388–3.382) | .807 |
| IUGR | 114 (37.3) | 19 (19.6) | 0.369 (0.323–1.265) | .199 | 125 (35.1) | 8 (17.4) | 0.389 (0.176–0.860) | .020* |
| Moderate to severe BPD | 40 (13.1) | 65 (67.0) | 0.503 (0.361–0.508) | < .001* | 67 (18.8) | 38 (82.6) | 0.467 (0.281–0.410) | < .001* |
| Postnatal steroid therapy | 34 (11.1) | 62 (63.9) | 0.512 (0.380–0.531) | < .001* | 60 (16.8) | 36 (78.3) | 0.399 (0.382–0.606) | < .001* |
| Duration of oxygen treatment (day) | 27.9 ± 26.1 | 81.2 ± 42.7 | 1.054 (1.042–1.066) | < .001* | 33.5 ± 31.3 | 98.6 ± 38.4 | 1.044 (1.031–1.057) | < .001* |
| Placental pathology | ||||||||
| CPI | 64 (21.0) | 26 (26.8) | 2.050 (1.170–3.592) | .038* | 71 (19.9) | 19 (46.3) | 2.825 (1.487–5.367) | .002* |
| CCA | 33 (10.8) | 10 (10.3) | 0.159 (0.691–3.152) | .159 | 36 (10.1) | 7 (15.2) | 1.590 (0.663–3.816) | .299 |
| Chronic deciduitis | 29 (9.5) | 12 (12.4) | 0.244 (0.874–3.872) | .244 | 33 (9.3) | 8 (17.4) | 2.061 (0.887–4.784) | .093 |
| VUE | 13 (4.3) | 2 (2.1) | 0.139 (0.042–2.501) | .139 | 15 (4.2) | 0 | 0.237 (0.013–4.413) | .335 |
| ACA | 53 (17.4) | 32 (33.0) | 2.079 (1.177–3.671) | .012* | 70 (19.7) | 15 (32.6) | 1.977 (1.012–3.862) | .046* |
| MVU | 118 (38.7) | 31 (32.0) | 0.744 (0.452–1.344) | .744 | 134 (37.6) | 15 (32.6) | 0.802 (0.417–1.540) | .507 |
| FVTOD | 29 (9.5) | 11 (11.3) | 0.806 (0.325–2.000) | .642 | 36 (10.1) | 4 (8.7) | 0.847 (0.287–2.498) | .763 |
Values are presented as mean ± SD or number (%).
ROP, retinopathy of prematurity; OR, odds ratio; CI, confidence interval; IUGR, intrauterine growth retardation; BPD, bronchopulmonary dysplasia; CPI, chronic placental inflammation; CCA, chronic chorioamnionitis; VUE, villitis of unknown etiology; ACA, amniotic fluid infection/inflammation; MVU, maternal vascular underperfusion; FVTOD, fetal vascular thrombo-occlusive disease; SD, standard deviation.
Table 4.
| Placental pathology |
No ROP |
Any stage ROP |
Type 1 ROP |
|||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | p-valuea | Adjusted OR (95% CI) | p-valuea | Adjusted OR (95% CI) | p-valuea | |
| CPI | 1.008 (0.492–2.068) | .982 | 1.647 (0.702–3.992) | .246 | 2.739 (1.112–6.749) | .029* |
| ACA | 0.811 (0.429–1.940) | .811 | 1.267 (0.531–3.023) | .594 | 0.833 (0.319–2.173) | .708 |
| MVU | 0.630 (0.315–1.261) | .630 | 2.036 (0.865–4.792) | .103 | 0.570 (0.211–1.539) | .267 |
| FVTOD | 0.815 (0.298–2.226) | .690 | 1.080 (0.360–3.240) | .126 | 0.424 (0.141–1.272) | .126 |
Chronic placental inflammation was statistically associated with type 1 ROP by multiple logistic regression analysis.
ROP, retinopathy of prematurity; OR, odds ratio; CI, confidence interval; CPI, chronic placental inflammation; ACA, amniotic fluid infection/inflammation; MVU, maternal vascular underperfusion; FVTOD, fetal vascular thrombo-occlusive disease.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download