Abstract
Background
Papillary thyroid carcinoma (PTC) is frequently accompanied by lymphocytic thyroiditis (LT). Some reports claim that Hashimoto’s thyroiditis (the clinical form of LT) enhances the likelihood of PTC; however, others suggest that LT has antitumor activity. This study was aimed to find out the relationship between the patterns of helper T cell (Th) cytokines in thyroid tissue of PTC with or without LT and the clinicopathological manifestation of PTC.
Methods
Fresh surgical samples of PTC with (13 cases) or without (10 cases) LT were used. The prognostic parameters (tumor size, extra-thyroidal extension of PTC, and lymph node metastasis) were analyzed. The mRNA levels of two subtypes of Th cytokines, Th1 (tumor necrosis factor α [TNF-α], interferon γ [IFN-γ ], and interleukin [IL] 2) and Th2 (IL-4 and IL-10), were analyzed. Because most PTC cases were microcarcinomas and recent cases without clinical follow-up, negative or faint p27 immunoreactivity was used as a surrogate marker for lymph node metastasis.
Results
PTC with LT cases showed significantly higher expression of TNF-α (p = .043), IFN-γ (p < .010), IL-4 (p = .015) than those without LT cases. Although the data were not statistically significant, all analyzed cytokines (except for IL-4) were highly expressed in the cases with higher expression of p27 surrogate marker.
The annual incidence of thyroid cancer is variable across the globe; for males, 1.2–2.6 per 100,000 and for females, 2.0–3.8 per 100,000. The Unites States, Japan, Sweden, and France have higher than average incidence rates [1,2]. In South Korea, the incidence of thyroid cancer is 0.88 per 100,000 in males and 6.68 per 100,000 in females. Among thyroid cancers, papillary thyroid carcinoma (PTC) is the most common (83.8%) [3]. Hence, the etiology, prognosis, and pathophysiology of PTC have been investigated extensively in research studies. Current studies focus on the molecular pathology (such as the roles of E-cadherin, c-Met, and epidermal growth factor receptor) and genetic alterations (such as RET/PTC, TRK, BRAF, and p53) of PTCs [4]. Most PTC cases are indolent and seldom behave aggressively, where aggressive behavior is characterized with frequent recurrences and metastases [5]. Shibru et al. [6] maintain that patients over the age of 45, with elevated expression levels of both cyclooxygenase 2 and vascular endothelial growth factor C, have a more aggressive PTC. Including this study, there have been many attempts to find prognostic factors for PTC. For instance, Kebebew et al. [7] has recently found that BRAF mutations are significantly associated with the aggressive behavior of thyroid cancer.
There have been many attempts to develop therapeutic vaccines for cancer [8]. Despite the many research studies on cancer immunotherapy, the role of immunity in the biological behavior of PTC is still poorly understood. Among the limited data collected on the influence of immunity on PTC behavior are findings indicating an association between the immunity and the good prognosis in cases of PTC concomitant with Hashimoto’s thyroiditis (HT) or lymphocytic thyroiditis (LT) [9,10].
It is traditionally assumed that tumor growth is suppressed by T-helper 1 cell (Th1) immunity and supported by T-helper 2 cell (Th2) immunity. However, even Th2 immunity can promote antitumor activity [11]. Th1 immunity activates cytotoxic CD8+ T lymphocytes (CTLs). Although CTLs can acquire antitumor immunity, some tumor cells escape CTL immune surveillance and survive. Hence, from a practical point of view, Th1 predominance in itself does not represent antitumor immunity. Th2 immunity provokes a humoral immune reaction, i.e., the antibody-synthesizing immunity. While components of the Th2 immunity, such as B cells and interleukin (IL) 10, make favorable conditions for tumor growth, tumor-infiltrating granulocytelinked Th2 immunity promotes antitumor activity [11].
Although there are relatively few reports about Th immunity and PTC, one by Mardente et al. [12] reported that a Th cytokine pattern from the peripheral blood of a patient with PTC with chronic LT has a predominantly Th2 immune reaction or mixed cell response. Intrathyroidal lymphocytes in HT are composed of both B cells and T cells, the majority of them are CD8+ T cells, which are cytotoxic to thyroid follicle cells [13]. PTCs are often associated with chronic LT and HT; for instance, Shull et al. [14] demonstrated that diffuse LT is associated with PTC without therapeutic histories. In addition, Mauras et al. [15] reported three cases of thyroid cancer with HT that did not have recurrent disease after a thyroidectomy. Therefore, it is difficult to determine which Th subtype immunity has superior antitumor activity over the other.
Several clinical trials have been conducted to induce cytotoxic immunity against thyroid cancers. Amino et al. [16] used saline homogenates of thyroid tumors, and Gerfo et al. [17] applied chemically altered thyroglobulins, but the clinical efficacy of these therapies have not been proven yet.
In the present work, we analyzed the pattern of Th immunity and investigated its relationship to the clinicopathological manifestation of PTC. With the current ease of early detection of PTCs, cases of advanced tumor stages are rare. Hence, we used a surrogate immunohistochemical marker, p27, to represent the possibility of lymph node metastasis, for it has been known that p27 expression is low in metastasizing PTC [18].
After Institutional Review Board (IRB) approval (protocol No. 08-0194), 23 patients from the Department of Pathology, Gangnam Severance Hospital, were enrolled in this study. The patients ranged in age from 35 to 59 years old, with a mean age of 47. Fresh surgical samples of PTC with (13 cases) or without (10 cases) LT were collected from the records in the Department of Pathology with the patients’ agreement and the formal permission of the IRB. The prognostic parameters (tumor size, extra-thyroidal extension of PTC, and lymph node metastasis) were analyzed. Among the subtypes of PTC samples in this group, 21 were of the most conventional type and two cases were follicular variants. The fresh thyroid tissues were divided into PTC and nontumor portions and sampled separately. The nontumor portions were used for measuring the mRNA quantities of the cytokines, and the PTC portions were subject to immunohistochemical staining of CIP1 (p21)/KIP1 (p27) for protein expression analysis, as described in more detail in later section.
The nontumor portions of the patient samples were used to measure the mRNA levels of the following cytokines: tumor necrosis factor α (TNF-α), interferon γ (IFN-γ), IL-2, IL-4, IL-10, and IL-1β. The 13 cases with concomitant PTC and LT were referred to as the objective group, and the 10 cases of PTC without LT were used as a reference or control group. Quantitative real-time polymerase chain reaction (PCR) was performed according to the manual of LightCycler 480 Real-Time PCR System (Roche Applied Science, Manheim, Germany).
The reference gene value (actin and 18S RNA) and IL-1β value were measured in every sample. Although it is known to be meaningless to compare the levels of cytokines from the same specimen, the expressed cytokine levels could be ranked and compared in this research via a quantitative real-time PCR method. Using the concept of relative quantification, the cytokine values were corrected for differences in quality and quantity by dividing the concentration of a target RNA by the concentration of a reference RNA in the same sample (relative ratio=concentration of target/concentration of reference). The most common way to compare expression levels of different samples is to designate one of the samples as calibrator, where all other samples are compared to this calibrator. For normalization of the final results, the target/reference ratio of each sample is divided by the target/reference ratio of the calibrator sample: calibrator normalized ratio=(sample; concentration of target/concentration of reference)/(calibrator; concentration of target/concentration of reference) [19]. In the present work, we applied the constitutively-expressed cytokine, IL-1β, as the calibrator. There were cytokine values expressed as “not detected.” This means that the reference gene was calculated, but the target gene could not be detected despite many PCR amplification processes. In these cases, we adjusted these “not detected” values to zero (0).
The immunohistochemistry (IHC) of the p27 protein expression in the PTC tumor tissue from all 23 cases was analyzed using paraffin-embedded tissue. Formalin-fixed paraffin-embedded sections (3 μm thick) were dewaxed in xylene and rehydrated through graded alcohols to water. Endogenous peroxidase activity was blocked in 3% hydrogen peroxide. Antigen retrieval was performed in citrate buffer (pH 6.0) within a microwave pressure cooker, and endogenous biotin detection was blocked with the Avidin-Biotin blocking kit (Vector Laboratories Inc., Burlingame, CA, USA).
Optimum primary antibody dilutions were predetermined, and appropriate positive control samples (tissues known to be positive for the immunohistochemical marker) and negative control samples (test tissue sections without the addition of primary antibody) were used for p27 (Novocastra, Newcastle upon Tyne, UK). The primary antibody incubation was diluted at 1:200 for 1 hour. After incubation, the slide was washed with phosphate buffered saline, and a secondary incubation was carried out with biotin anti-mouse/anti-rabbit IgG followed by streptavidin-HRP (Signet Pathology System, Dedham, MA, USA) for 30 minutes. The immunoreaction was revealed by incubation in 3-amino-9-ethylcarbazole. The slides were counterstained with hematoxylin and mounted in balsam.
When p27 immunoreaction was interpreted, only the nuclear staining on PTC cells was regarded as positive. Its measurement was evaluated by the following two-tiered grading system: low grade (negative staining or faint staining in less than 30% of the tumor cells) and high grade (positive staining in more than 30% of the tumor cells) (Fig. 1) [20]. Nuclear p27 immunoreactivity was considered altered when the expression was less than 30% of previously published, clinically relevant levels. In 21 cases, the results of IHC were summarized in two groups: the cases with LT (n=13) and the cases without LT (n=8). Two cases were not performed, for the IHC sections for p27 were devoid of tumor tissue.
The Pearson chi-square test was used to examine the relationships of cytokines (TNF-α, IFN-γ, IL-2, IL-4, IL-10, and IL-1β) and p27 expression with the clinicopathological characteristics. All reported p-values were 2-sided, and the significance was set at .05. All statistical tests were performed with SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA).
The subtypes of the PTCs with LT were determined to be the most conventional (11/13, 84.61%), and those of only two cases were follicular variants (2/13, 15.38%). The tumor sizes ranged from 0.2 to 1.1 cm, with an average of 0.55 cm. Of the 13 cases of this type, six cases showed extrathyroidal tumor extension (ET) (6/13, 46.15%), and two cases presented with lymph node metastasis (2/13, 15.38 %).
The subtypes of the PTCs with LT were all conventional (10/10, 100%). Three cases with ET were present (3/10, 30%). Tumor sizes ranged from 0.1 to 1.5 cm, with an average of 0.61 cm. There were three cases with lymph node metastasis (3/10, 30%).
In all 23 cases, the cytokine immune profiles were summarized into two groups: the cases with LT (n=13) and the cases without LT (n=10). The cytokines could have originated from inflammatory cells, thyroid tissue, and endothelial cells. In this research, the tissues were from the thyroid tissue near PTC.
After manipulating the data using the concept of a calibrator with IL-1β, each sample of PTC with LT was compared (Table 1). Among the six cytokines analyzed, TNF-α, IL-4, and IFN-γ were relatively well expressed. Based on these factors, the cytokine expression in the PTC samples with LT represented a mixed Th1 (TNF-α and IFN-γ) and Th2 (IL-4 and IL-10) immunity.
After manipulating data using the concept of a calibrator with IL-1β, each sample of PTC without LT was compared (Table 2). In contrast to the cases of PTC with LT, the cases without LT frequently exhibited nondetectable cytokine levels. Among the six cytokines analyzed, the expression levels of IFN-γ, IL-10, and IL-2 levels were too low to be measured. Based on these data, the cytokines expressed in PTC without LT represented a mixed Th1 (TNF-α) and Th2 (IL-4 and IL-1β) immunity.
The prognostic parameters (tumor size, ET, and lymph node metastasis) were not statistically related to LT, but the cases without LT had a tendency for lymph node metastasis (Table 3).
The cases with LT had enhanced expression of the cytokines (TNF-α, IFN-γ, IL-4, IL-10, and IL-2) compared to the samples of PTC without LT. Among the five cytokines, the expression levels of TNF-α, IFN-γ, and IL-4 were significantly higher in the cases with LT than in those without LT (p<.05).
In terms of p27 as a surrogate marker for lymph node metastasis, the degree of p27 expression was not correlated with lymph node metastasis in this study; high-grade expression of p27 had a tendency to occur in PTC cases with LT. In contrast, lymph node metastasis occurred slightly more frequently in PTC cases without LT. Conclusively, the degree of p27 expression did not show any correlation with lymph node metastasis in this study (p=.15) (Table 4).
Due to this unexpected finding, which revealed in the insufficiency of the PTC cases without LT to serve as a reference group, this study adopted a new goal to use the PTC cases with LT to analyze the relationship between cytokine immune profiles and prognostic parameters. In addition, the PTC cases in this study were mostly microcarcinomas, which might represent the incipient phase of the tumors. The incipient phase of tumors must always have a limitation in representing proper tumor staging, such as tumor size, nodal metastasis, and tumor extension. Hence, in this study, p27 was used as a surrogate marker of lymph node metastasis in the cases of microcarcinoma (as an incipient carcinoma).
Therefore, the statistical analysis of this profiles solely made use of the PTC cases with LT. Except for the IL-4 cytokine, the expression levels of the cytokines TNF-α, IFN-γ, and IL-10 were lower in the cases with low-grade expression of p27 than those with high-grade expression of p27, but there was no statistical significance (Table 5). The cases with ET had a tendency to demonstrate higher levels of cytokine expression than those with intrathyroidal tumor confinement (Table 6). Again, there was no statistical significance.
In summary, although the data were not statistically significant, the trends follow a pattern where higher cytokine levels were present in the cases with high grade expression of p27 (except for IL-4) and ET.
This study revealed the presence of mixed Th1 (IFN-γ, TNF-α, and IL-2) and Th2 (IL-4 and IL-10) immunity in lymphocytes in cases of PTC with LT.
Although there is no statistical significance with a limited case number, this study could infer some meaningful results.
In the cases with LT, the higher expression of cytokine levels had a tendency to be associated with high p27 immuno-reactivity (Table 5). Furthermore, except for IL-4, all cytokines were decreased in the cases with LT and p27 underexpression, which represents the increased possibility of lymph node metastasis. Because IL-4 is associated with aggressive PTC, the fact that all cytokine except IL-4 were highly expressed in the cases with LT and increased p27 expression supports the hypothesis that high expression of cytokines except IL-4 may contribute to anticancer effect on the PTC in terms of lymph node metastasis [21].
In contrast, the cases with ET revealed the higher expression of cytokines than those without ET. Based on these results, we propose that the higher expression of cytokines TNF-α, IFN-γ, IL-2, and IL-1 might inhibit nodal metastasis but not inhibit (or possibly enhance) the extrathyroidal extension. In view of helper T cell immunity, mixed Th1 and Th2 immunity seems to play a role in anticancer activity by inhibiting lymph node metastasis.
There have been several studies on the immune profiles of thyroiditis. Among them, Phenekos et al. [22] reported that HT and Graves’ disease have two different helper T cell immunities. In their report, with the preferential expression of IL-2, IFN-γ, IL-12, and IL-18, a Th1 pattern of immune response which is characteristic of cellular immunity, is dominant in HT. In contrast, Ajjan et al. [23] reported a mixed Th1 and Th2 immune response in HT cases. They reported that reverse transcription polymerase chain reaction results showed both Th1 and Th2 immunity. The findings in our study corroborate these results.
Our findings demonstrated that IL-4 expression, together with other cytokines, has a tendency to be higher in the cases with ET. This may mean that IL-4 contributes to tumor extension. In contrast, in the cases with underexpression of p27, which may represent lymph node metastasis, IL-4 was higher than other cytokines. This may imply that IL-4 contributes to the lymphatic spread of the tumor, a concept that is supported by Vella et al. [21] who demonstrated that IL-4 levels were augmented in aggressive PTCs. They suggested that PTC cells receive protection from apoptosis by IL-4 production in the activated T lymphocytes of thyroid glands. To determine which of these conflicting results in terms of both nodal metastasis and capsular tumor extension are correct, further study may be necessary to disclose the role of IL-4 in PTC biological behavior.
A previous work by Yip et al. [24] demonstrated with in vitro tests that IL-1β was an anticancer factor, which suppressed the proliferation and reduced the invasive potential of human PTC cells. In this study, IL-1β was found to be constitutively expressed in all cases and was used as a calibrator to compare cytokine levels among the cases. Unfortunately, due to its use as a calibrator in this study, IL-1β expression levels in each case could not be compared.
It is generally accepted that there is a beneficial relationship between chronic LT and the biological behavior of PTC, which results in improved prognoses. Paulson et al. [25] suggested that chronic LT might have a protective role in tumor spread. Supporting this evidence, Mitsiades et al. [26] reported that Th1 cytokines, such as IFN-γ and TNF-α, increase the sensitivity of both normal and neoplastic thyrocytes to FasL and TRAIL, which lead to apoptosis. Furthermore, Ahn et al. [27] established that HT was associated with PTC, as was chronic inflammation with cancer in other locations. They also mentioned that the coexistence of HT in PTC cases introduced favorable clinical outcomes compared with those of PTC without HT [27]. Corroborating this idea, Yoon et al. [28] determined that patients with PTC and chronic LT had smaller tumor sizes, a lower incidence of capsular invasion, and a significantly lower incidence of lymph node metastases compared to patients without chronic LT. Several studies have also indicated that antithyroid antibodies are able to recognize these malignant cells and destroy them in the same way that they destroy normal follicular cells, contributing to the low rate of clinical progression of these lesions [29,30].
The p27 protein was first identified as an inhibitor of cyclin E/CDK2 complexes during transforming growth factor β–induced G1 arrest [31]. Phosphorylation is the mechanism primarily used for regulating p27 activity. The p27 protein possesses multiple tyrosine, serine, or threonine phosphorylation sites. The inhibitory roles of p27 towards cyclin/CDK complexes are weakened by phosphorylation directed by certain signal transduction pathways [32]. The current model delineates that p27 suppresses tumorigenesis by inhibiting cyclin/CDK activity in the nucleus, but it exerts other functions in the cytoplasm that are potentially oncogenic [33].
There have been several clinical studies describing the relationship between the expression of p27 and lymph node metastasis. Karlidag et al. [18] reported that p27 expression in nonmetastasizing PTC was lower than that in normal thyroid tissue and higher than that in metastasizing PTC.
Our IHC analysis showed that p27 expression did not demonstrate any relationship to lymph node metastasis in PTC with LT, but this may be attributed to the early stage of the tumors, i.e., our study mainly included patients with microcarcinomas. There may be a chance that even PTC cases with low expression of p27 were too incipient to reveal lymph node metastasis. With this limitation in the evaluation of the relationship between cytokine levels and nodal metastasis, the common p27 marker was used as a surrogate marker to represent the possibility of nodal metastasis in early phase PTCs. As aforementioned, after focusing on the cases with LT, the cases with low-grade expression of p27 tended to be associated with lower levels of cytokines than those with high-grade expression of p27. This may implicate that the cases with lower levels of mixed Th1 and Th2 cytokines have a higher probability of having lymph node metastasis.
Considering the low cytokine expression in the cases with underexpression of p27 (except for IL-4), our results indicate that mixed Th1 and Th2 immune cytokines have a tendency toward anticancer effects in terms of lymph node metastasis. To predict tumor prognosis from cytokine levels, further studies determining the absolute values and IHC of cytokines will be necessary.
ACKNOWLEDGMENTS
This work is funded by a grant of 2015 Korean Thyroid Association. We thank Su Jin Jeong. Without her endeavors and precise experimentation, the data for this research would not be as accurate.
REFERENCES
1. Kuijpens JL, Coebergh JW, van der Heijden LH, Kruis H, Ribot JG, de Rooij HA. Thyroid cancer in Southeastern Netherlands, 1970-1989: trends in incidence, treatment and survival. Ned Tijdschr Geneeskd. 1994; 138:464–8.
2. Laurberg P, Nøhr SB, Pedersen KM, et al. Thyroid disorders in mild iodine deficiency. Thyroid. 2000; 10:951–63.

3. Ministry for Health, Welfare and Family Affairs; Korea Central Cancer Registry. Cancer incidence in Korea 1999-2002. Goyang: Korea Central Cancer Registry;2008.
4. Sobrinho-Simoes M, Preto A, Rocha AS, et al. Molecular pathology of well-differentiated thyroid carcinomas. Virchows Arch. 2005; 447:787–93.

5. Siironen P, Louhimo J, Nordling S, et al. Prognostic factors in papillary thyroid cancer: an evaluation of 601 consecutive patients. Tumour Biol. 2005; 26:57–64.

6. Shibru D, Chung KW, Kebebew E. Recent developments in the clinical application of thyroid cancer biomarkers. Curr Opin Oncol. 2008; 20:13–8.

7. Kebebew E, Weng J, Bauer J, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007; 246:466–70.
8. Finke LH, Wentworth K, Blumenstein B, Rudolph NS, Levitsky H, Hoos A. Lessons from randomized phase III studies with active cancer immunotherapies--outcomes from the 2006 meeting of the Cancer Vaccine Consortium (CVC). Vaccine. 2007; 25 Suppl 2:B97–109.
9. McConahey WM, Hay ID, Woolner LB, van Heerden JA, Taylor WF. Papillary thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1986; 61:978–96.

10. Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A, Shah JP. Coexistent Hashimoto’s thyroiditis with papillary thyroid carcinoma: impact on presentation, management, and outcome. Surgery. 1999; 126:1070–6.

11. Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens. 2007; 70:1–11.

12. Mardente S, Lenti L, Lococo E, et al. Phenotypic and functional characterization of lymphocytes in autoimmune thyroiditis and in papillary carcinoma. Anticancer Res. 2005; 25:2483–8.
13. Baker JR Jr, Fosso CK. Immunological aspects of cancers arising from thyroid follicular cells. Endocr Rev. 1993; 14:729–46.

14. Shull JH, Sharon N, Victor TA, Scanlon EF. Thyroid carcinoma: immunology, irradiation, and lymphocytic infiltration. Arch Surg. 1979; 114:729–31.
15. Mauras N, Zimmerman D, Goellner JR. Hashimoto thyroiditis associated with thyroid cancer in adolescent patients. J Pediatr. 1985; 106:895–8.

16. Amino N, Pysher T, Cohen EP, Degroot LJ. Immunologic aspects of human thyroid cancer: humoral and cell-mediated immunity, and a trial of immunotherapy. Cancer. 1975; 36:963–73.
17. Gerfo PL, Feind C, Weber C, Ting W. Immunotherapy of thyroid cancer by induction of autoimmune thyroiditis. Surgery. 1983; 94:959–65.
18. Karlidag T, Cobanoglu B, Keles E, et al. Expression of Bax, p53, and p27/kip in patients with papillary thyroid carcinoma with or without cervical nodal metastasis. Am J Otolaryngol. 2007; 28:31–6.

19. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001; 29:e45.

20. Resnick MB, Schacter P, Finkelstein Y, Kellner Y, Cohen O. Immunohistochemical analysis of p27/kip1 expression in thyroid carcinoma. Mod Pathol. 1998; 11:735–9.
21. Vella V, Mineo R, Frasca F, et al. Interleukin-4 stimulates papillary thyroid cancer cell survival: implications in patients with thyroid cancer and concomitant Graves’ disease. J Clin Endocrinol Metab. 2004; 89:2880–9.

22. Phenekos C, Vryonidou A, Gritzapis AD, Baxevanis CN, Goula M, Papamichail M. Th1 and Th2 serum cytokine profiles characterize patients with Hashimoto’s thyroiditis (Th1) and Graves’ disease (Th2). Neuroimmunomodulation. 2004; 11:209–13.

23. Ajjan RA, Watson PF, McIntosh RS, Weetman AP. Intrathyroidal cytokine gene expression in Hashimoto’s thyroiditis. Clin Exp Immunol. 1996; 105:523–8.

24. Yip I, Pang XP, Berg L, Hershman JM. Antitumor actions of interferon-gamma and interleukin-1 beta on human papillary thyroid carcinoma cell lines. J Clin Endocrinol Metab. 1995; 80:1664–9.

25. Paulson LM, Shindo ML, Schuff KG. Role of chronic lymphocytic thyroiditis in central node metastasis of papillary thyroid carcinoma. Otolaryngol Head Neck Surg. 2012; 147:444–9.

26. Mitsiades CS, Poulaki V, Mitsiades N. The role of apoptosis-inducing receptors of the tumor necrosis factor family in thyroid cancer. J Endocrinol. 2003; 178:205–16.

27. Ahn D, Heo SJ, Park JH, et al. Clinical relationship between Hashimoto’s thyroiditis and papillary thyroid cancer. Acta Oncol. 2011; 50:1228–34.

28. Yoon YH, Kim HJ, Lee JW, Kim JM, Koo BS. The clinicopathologic differences in papillary thyroid carcinoma with or without co-existing chronic lymphocytic thyroiditis. Eur Arch Otorhinolaryngol. 2012; 269:1013–7.

29. Boi F, Lai ML, Marziani B, Minerba L, Faa G, Mariotti S. High prevalence of suspicious cytology in thyroid nodules associated with positive thyroid autoantibodies. Eur J Endocrinol. 2005; 153:637–42.

30. Lucas SD, Karlsson-Parra A, Nilsson B, et al. Tumor-specific deposition of immunoglobulin G and complement in papillary thyroid carcinoma. Hum Pathol. 1996; 27:1329–35.

31. Larrea MD, Liang J, Da Silva T, et al. Phosphorylation of p27Kip1 regulates assembly and activation of cyclin D1-Cdk4. Mol Cell Biol. 2008; 28:6462–72.
32. Lee J, Kim SS. The function of p27 KIP1 during tumor development. Exp Mol Med. 2009; 41:765–71.
Fig. 1.
p27 immunoreactivity (nuclear staining) on papillary thyroid carcinoma cells. Two-tiered grading system reveals a low grade (A, negative staining or faint staining less than 30% of tumor cells) and a high grade (B, positive staining more than 30% of tumor cells).
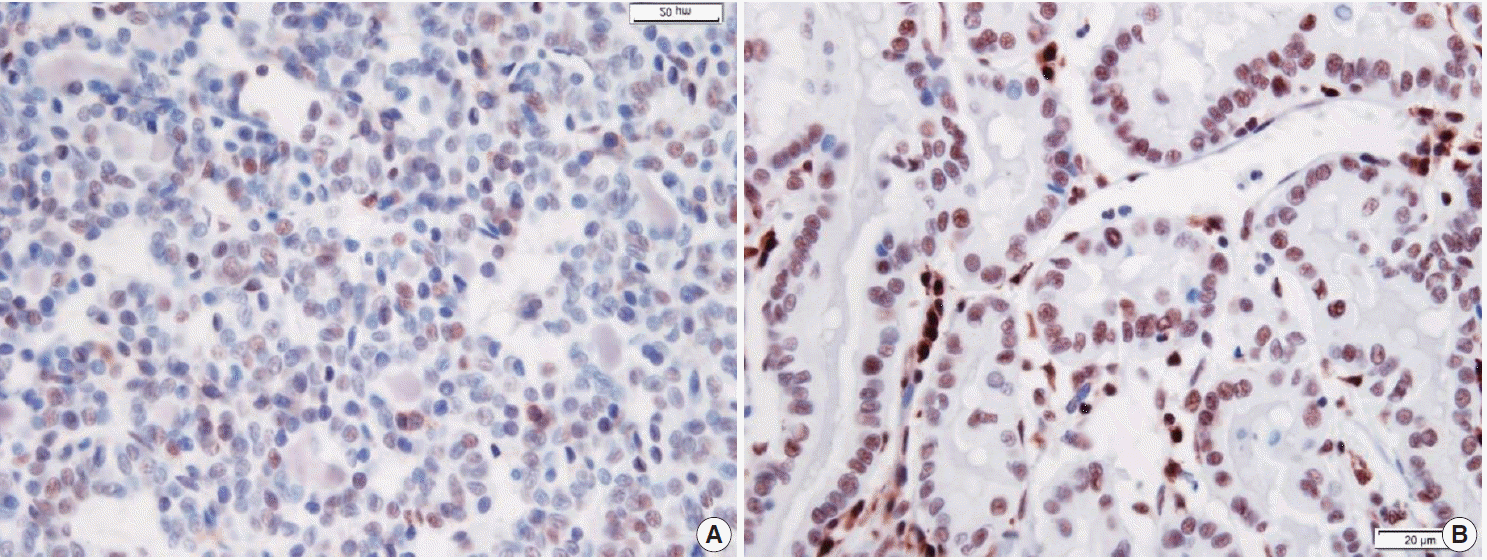
Table 1.
Cytokine and immune profiles of the cases with lymphocytic thyroiditis (n = 13)
Table 2.
Cytokine and immune profiles of the cases without lymphocytic thyroiditis (n = 10)
Table 3.
Relationship between clinical parameters and the cases with lymphocytic thyroiditis
Values are presented as median (range) or number (%).
The cytokine unit value is the ratio of each cytokine to IL-1β.
LT, cases with lymphocytic thyroiditis; Non-LT, cases without lymphocytic thyroiditis; LG p27, low-grade expression of p27 immunohistochemistry; TNF-α, tumor necrosis factor α; IL, interleukin; IFN-γ, interferon γ.
Table 4.
Relationship between p27 immunopositivity and lymph nodal metastasis
| Variable | Lymph nodal metastasis (n = 5) | Without lymph nodal metastasis (n = 18) |
|---|---|---|
| LG p27 (n = 14) | 3/5 (60) | 11/18 (61.11) |
| HG p27 (n = 7) | 1/5 (20) | 6/18 (33.33) |
Table 5.
Relationship between p27 immunopositivity and the cytokine immune profiles in the cases with lymphocytic thyroiditis (n = 13)
Table 6.
Relationship between cytokine levels and tumor extension in the cases with lymphocytic thyroiditis (n = 13)




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download