Abstract
Purpose
Hepatocellular carcinoma (HCC) is rare in HCV patients without cirrhosis, and little is known about the postoperative results of these patients. The present study compares the outcomes of cirrhotic and non-cirrhotic groups after liver resection (LR) in solitary HCV-related HCC patients and identifies risk factors for prognosis according to the presence or absence of cirrhosis in these patients.
Methods
Two hundred and 7 adult hepatectomy patients with treatment-naïve solitary HCV-related HCC were identified prospectively at our institution between July 2005 and May 2019.
Results
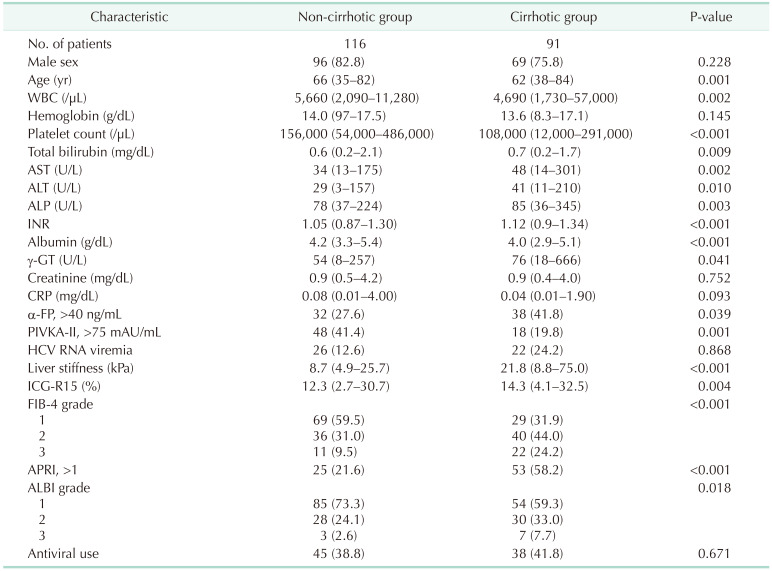
The non-cirrhotic group had better liver function than the cirrhotic group based on platelet count, liver function tests, liver stiffness measurement, and indocyanine green retention rate at 15 minutes but were older than the cirrhotic group. Consistently, noninvasive markers in the cirrhotic group were significantly higher than in the non-cirrhotic group. The cumulative disease-free survival and overall survival in the non-cirrhotic group were significantly higher than in the cirrhotic group. HCC recurrence was related to major LR and α-FP of >40 ng/mL and death was related to long hospitalization and α-FP of >40 ng/mL in multivariate analysis. Noninvasive markers and the presence of cirrhosis were not related to HCC recurrence or death in multivariate analyses.
The prevalence of chronic HBV and chronic HCV in Korea was 3.4% and 0.3% in 2016–2017, respectively [1]. HCV infection is a slowly progressing disease characterized by persistent hepatic inflammation. Chronic HCV infection leads to cirrhosis in approximately 10%–20% of patients over 20–30 years [2]. The incidence of hepatocellular carcinoma (HCC) among HCV-infected patients is between 1% and 3% over 30 years [3]. In addition, some HCV patients with Ishak grade 3 or 4 fibrosis develop HCC [2]. Therefore, the incidence of HCC in non-cirrhotic HCV patients ranges from 4.4% to 10.6% [4]. Although several studies have described the characteristics and outcomes of HCC in the non-cirrhotic liver [56], there are limited data on HCC in patients with chronic HCV infection in the absence of cirrhosis.
Liver resection (LR) has been used as curative treatment for solitary HCC due to its demonstrated impact on prognosis and its low morbidity and mortality rates if liver function is preserved [7]. Hepatic fibrosis and cirrhosis often accompany impaired liver function and are associated with postoperative liver dysfunction, which increases morbidity and mortality after LR in HCC patients [8]. Preoperative assessment of hepatic functional reserve is important to individualize treatment strategies for HCC. Several noninvasive scoring systems for chronic liver disease have been established based on routine laboratory parameters. The fibrosis-4 (FIB-4) grade, albumin-bilirubin (ALBI) grade, and AST-to-platelet count ratio index (ARPI) are 3 alternative biomarkers of liver fibrosis and cirrhosis [9101112]. Several previous studies have investigated the accuracy of the FIB-4 grade, ALBI grade, and ARPI to predict the short-term outcomes of LR in HCC patients [91213141516]. However, the APRI and FIB-4 index cannot predict fibrosis in HBV patients [17].
The incidence of HCC recurrence remains high after curative LR [618]. Liver fibrosis is an important risk factor for multicentric carcinogenesis [8]. Noninvasive liver fibrosis models have not been useful for predicting HCC recurrence and death in patients with HCV-related HCC who are scheduled for LR.
The present study compares the outcomes of cirrhotic and non-cirrhotic groups after LR in solitary HCV-related HCC patients and identifies risk factors for prognosis according to the presence or absence of cirrhosis in these patients.
The HCC registry of Samsung Medical Center includes newly diagnosed HCC patients who received primary care at Samsung Medical Center. Our institution prospectively has been collecting patient data since January 2005. Detailed information about the registry has been provided previously [19]. This study was approved by the Institutional Review Board of Samsung Medical Center (SMC-2020-10-110) and the informed consent of the participants was exempted.
Patients with preserved liver function, treatment-naïve solitary HCC, and positive anti-HCV immunoglobulin G (IgG) were included. Patients with any of the following features were excluded: Child-Pugh class B or C, HBV infection, alcoholic liver disease, ruptured HCC, a history of portal vein embolization, mixed HCC and cholangiocarcinoma, serosal involvement, synchronous operations due to other malignant diseases, insufficient data, and those who were lost to follow-up after surgery. R1 resection was defined as free resection margin less than 1mm from HCC and was excluded from this study. In addition, HCC patients with positive anti-HCV IgG were excluded if they consumed >60 g/day of alcohol [20]. Ultimately, 207 adult hepatectomy patients with HCV-related HCC who attended our institution between July 2005 and May 2019 were included.
HCC and cirrhosis in the background liver were diagnosed in the pathologic report after LR. Postoperative mortality was defined as death within 90 days of surgery or during the hospital stay depending on which was longer. The ALBI score, FIB-4 index, and APRI were calculated using the following formulas: ALBI score = –0.085 × (albumin [g/L]) + 0.66 × log10(bilirubin [mmol/L]); FIB-4 = age [years] × AST [U/L]/platelet count [109/L]/ALT [U/L]1/2); APRI = ([AST/upper limit of normal]/platelet count [109/L]) × 100. The ALBI score was stratified as follows: ALBI grade 1 (≤–2.60); grade 2 (>–2.60 to ≤–1.39); or grade 3 (>–1.39). The FIB-4 index was stratified as follows: grade I (<3.25); grade 2 (3.26–6.70); or grade 3 (>6.70) [10121421].
The demographic, preoperative, surgical, and pathologic data were collected prospectively from the electronic medical records. Demographic characteristics were age, sex, and body mass index. Transient elastography was performed before liver surgery to measure liver stiffness. The surgical data collected were blood loss, intraoperative RBC transfusion, major or minor LR, anatomical LR, operative time, and hospitalization. Minor LR was defined as removal of one or 2 segments, while major LR involved removal of 3 or more segments. The following postoperative pathological variables were measured: tumor size, tumor grade, encapsulation, microvascular invasion, intrahepatic metastasis, cirrhosis, and free resection margin from the tumor. None of the hepatectomy patients received postoperative adjuvant therapy before HCC recurrence. The procedures used for surveillance after LR have been described previously [622].
The surgical procedures practiced by our institution have been detailed previously [622]. All surgical resections were performed by 4 highly experienced liver and transplant surgeons (JMK, GSC, CHDK, and JWJ). All decisions regarding transfusions were made based on clinical assessment of the anesthetist. No specific laboratory values (including hemoglobin or hematocrit) were used to prompt blood transfusion. The Jackson-Pratt drain was removed if the volume of drainage was ≤400 mL for 3 consecutive days. Patients were discharged once total bilirubin, AST, and ALT were within twice the normal upper limit.
Continuous variables are reported as medians and ranges. Nominal variables are reported as numbers and percentages. Continuous variables were compared using the Mann-Whitney U-test. Nominal scales were compared using Fisher exact test. The disease-free survival (DFS) and overall survival (OS) were calculated based on the duration from the start date of LR to the date when HCC recurrence or death occurred, or to the date of the last follow-up visit. The differences between the DFS and OS across the 2 groups were assessed using the Kaplan-Meier survival method. The prognostic factors for HCC recurrence and death in univariate analysis were determined using the Cox-regression hazard model. Based on these results, variables with a P-value of <0.05 were included in multivariate analyses. All statistical analyses were performed using IBM SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA). A P-value of <0.05 was considered statistically significant. All of the statistical tests were evaluated as 2-tailed.
We identified all adult HCV-infected patients with pathologically confirmed HCC between 2005 and 2019 (n = 207). During the study period, 116 patients (56.0%) had HCC in the absence of cirrhosis. The remaining 91 patients (44.0%) had cirrhosis in the background liver. The baseline characteristics are summarized in Table 1. The median age, WBC counts, and platelet counts in the non-cirrhotic group were significantly higher than in the cirrhotic group. Conversely, the median total bilirubin, AST, ALT, ALP, international normalized ratio (INR),γ-GT, indocyanine green retention rate at 15 minutes (ICG-R15), and liver stiffness measurement (LSM) were significantly higher in the cirrhotic group than in the non-cirrhotic group. The median albumin level in the cirrhotic group was lower than that in the non-cirrhotic group. The median α-FP in the non-cirrhotic group was lower than that in the cirrhotic group (9.4 ng/mL vs. 22.9 ng/mL, P = 0.011). The median protein induced by vitamin K absence or antagonist-II in the non-cirrhotic group was higher than that in the cirrhotic group (62 mAU/mL vs. 35 mAU/mL, P = 0.002). The proportion of patients with HCV viremia was 22.4% (n = 26) in the non-cirrhotic group and 24.2% (n = 22) in the cirrhotic group. Preoperative FIB-4 grade, ALBI grade, and APRI in the cirrhotic group were all significantly higher than those in the non-cirrhotic group. Forty-five patients (38.8%) in the non-cirrhotic group received antiviral therapy.
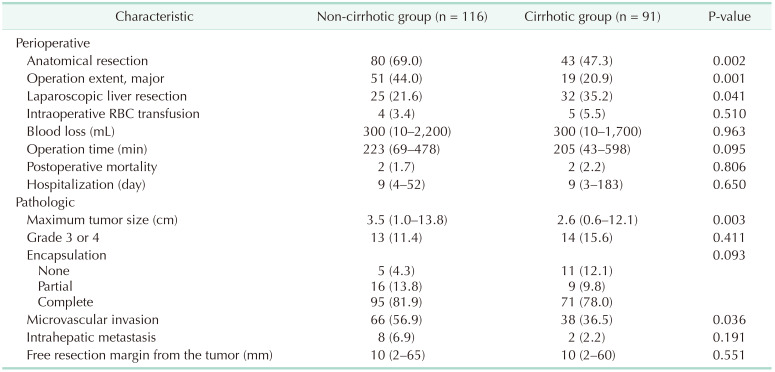
The perioperative and pathologic characteristics are outlined in Table 2. The proportions of anatomical LR and major LR in the non-cirrhotic group were significantly higher than those in the cirrhotic group. The incidence of laparoscopic LR in the non-cirrhotic group was lower than that in the cirrhotic group. There were no significant differences in amount of blood transfused during the operation, blood loss, operation time, postoperative mortality, or hospitalization between the 2 groups.
The median tumor size and presence of microvascular invasion were significantly greater in the non-cirrhotic group than those in the cirrhotic group. In the non-cirrhotic group, 78.4% (n = 71) of patients had septal fibrosis and 20.7% (n = 19) had periportal fibrosis, while one patient had no fibrosis. There were no significant differences in the proportions of patients with pathological tumor grade 3 or 4, encapsulation, intrahepatic metastasis, or free resection margin from the tumor between the 2 groups. There was no in-hospital mortality in either group.
The median follow-up duration was 47 months (range, 5.2–158.9 months) in the non-cirrhotic group and 35 months (range, 4.8–156.1 months) in the cirrhotic group. During the postoperative follow-up period, HCC recurred in 37 patients (31.9%) in the non-cirrhotic group and 36 (39.6%) in the cirrhotic group. The cumulative DFS rates at 1-year, 3-year, and 5-year were 87.4%, 72.9%, and 62.4% in the non-cirrhotic group and 78.5%, 62.0%, and 50.9% in the cirrhotic group, respectively (P= 0.045) (Fig. 1). Extrahepatic recurrence occurred in one case in the non-cirrhotic group and 2 cases in the cirrhotic group. The remaining recurrent HCC patients experienced intrahepatic recurrence. For recurrent HCC, transarterial chemoembolization (n = 13), radiofrequency ablation (RFA, n = 11), transcatheter arterial chemoembolization (TACE) and RFA (n = 4), re-resection (n = 6), radiation (n = 2), and systemic therapy (n = 1) were performed in the non-cirrhotic group. In the cirrhotic group, TACE (n = 14), RFA (n = 11), TACE and RFA (n = 3), re-resection (n = 1), radiation (n = 2), systemic therapy (n = 2), and others (n = 1) were performed.
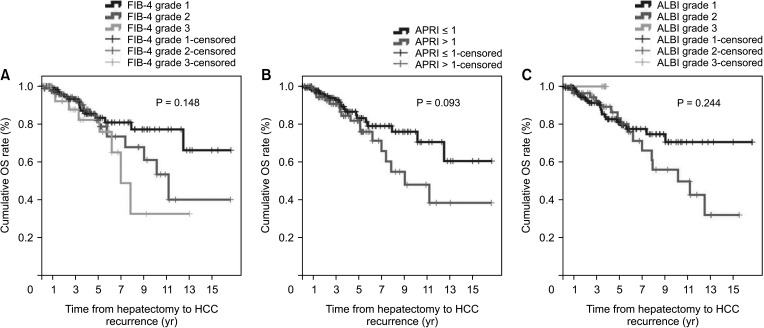
During the follow-up period, 20 patients (17.2%) in the non-cirrhotic group and 27 patients (29.7%) in the cirrhotic group died. The cumulative OS rates at 1-year, 3-year, and 5-year were 100%, 93.2%, and 85.6% in the non-cirrhotic group and 96.3%, 91.4%, and 81.3% in the cirrhotic group, respectively (P = 0.044) (Fig. 2). OS in the cirrhotic group abruptly decreased from 5 to 10 years after LR compared to that in the non-cirrhotic group. The causes of mortality were HCC (n = 13, 11.2%), gallbladder cancer (n = 1, 0.9%), lung cancer (n = 1, 0.9%), hepatic failure (n = 1, 0.9%), and unknown (n = 4, 3.4%) in the non-cirrhotic group and HCC (n = 21, 23.1%), hepatic failure (n = 1, 1.1%), peritonitis (n = 1, 1.1%), and unknown (n = 2, 2.2%) in the cirrhotic group. This outcome is similar to that observed in patients with poor liver function according to FIB-4 grade, ALBI grade, and APRI, who showed decrease in DFS and OS after 5 years after curative LR (Figs. 3 and 4).
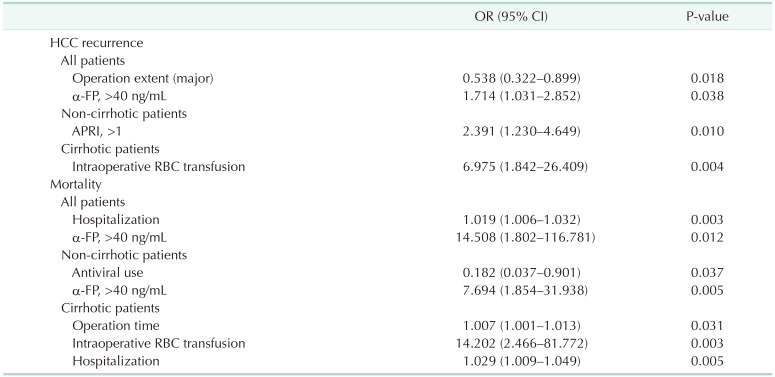
Significant factors closely associated with HCC recurrence and death after LR of HCV-related HCC patients were analyzed and are summarized in Table 3 (Supplementary Tables 1, 2, 3). Major LR and α-FP of >40 ng/mL were associated independently with HCC recurrence in all patients. In subgroup analysis, APRI of >1 in the non-cirrhotic group and intraoperative RBC transfusion in the cirrhotic group was the only predictive factors of HCC recurrence after curative LR. Long hospitalization after LR and α-FP of >40 ng/mL were associated closely with patient death in all patients. In subgroup analysis, antiviral use and α-FP of >40 ng/mL were associated closely with patient death in the non-cirrhotic group. In the cirrhotic group, long operation time, intraoperative RBC transfusion, and prolonged hospitalization were predisposing factors for patient death.
Patients infected with HCV often develop HCC, but development of this complication in the absence of liver cirrhosis is rare [523]. These results suggest that other etiological factors are more important than presence or absence of liver cirrhosis. None of the cases in the current study showed histological evidence of steatohepatitis, irritating molecules, or iron accumulation, which are recognized factors in the development of liver injury and HCC.
We compared the baseline, perioperative, and postoperative characteristics between the non-cirrhotic and cirrhotic treatment-naïve solitary HCV-related HCC patients after curative LR. We found that non-cirrhotic patients generally were older than cirrhotic patients, which was consistent with a previous finding [5]. We also found that platelet counts and serum albumin level, which reflect liver function, were significantly lower in the cirrhotic group than in the non-cirrhotic group. In contrast, the liver function tests, INR, LSM, and ICG-R15 were all significantly higher in the cirrhotic group than in the non-cirrhotic group.
The noninvasive scoring models that are used to measure hepatic function impairment, including the FIB-4 grade and APRI, were developed in chronic HCV patients [924]. The ALBI grade has been also proposed as a simple and objective method to assess functional reservoirs in HCC [12]. These noninvasive fibrosis markers have been shown to predict accurately the presence of cirrhosis and severe fibrosis when validated with liver biopsy findings [1020]. Our study also demonstrated that, as expected, FIB-4 grade, ALBI grade, and APRI were higher in the cirrhotic group than in the non-cirrhotic group.
Despite a larger tumor burden, the preserved function of the non-cirrhotic liver generally allows large LRs to be performed safely. In our study, the proportions of anatomical LR and major LR performed in the non-cirrhotic group were significantly higher than those performed in the cirrhotic group. In addition, the median tumor size in the non-cirrhotic group was higher than that in the cirrhotic group. However, intraoperative RBC transfusion rate, operation time, and length of hospitalization were similar between the 2 groups.
The perioperative mortality (0%–6%) and morbidity (8%–40%) of hepatectomy patients are low [23]. Despite the presence of chronic HCV disease, significant advancements in surgical technique and perioperative care have reduced in-hospital mortality after LR in HCC patients. There was no in-hospital mortality in our study. In cases of long hospital stays after LR, ascites may occur and liver function may not recovery completely. These findings suggest that the remnant liver function has deteriorated.
A major obstacle for HCC treatment is the high frequency of recurrence even after complete LR [25]. The incidence or pattern of HCC recurrence and prognosis depend on the background liver function and tumor characteristics [618]. Large tumor size, microvascular invasion, and cirrhosis are important contributing factors for tumor recurrence and mortality. However, treatment-naïve solitary HCV-related HCC in our study showed that preoperative α-FP level and perioperative factors of intraoperative RBC transfusion, operation time, and hospitalization were more important than pathologic factors for predicting HCC recurrence and death.
α-FP is considered a prognostic tumor marker for HCC and is involved in proliferation, apoptosis, and autophagy of hepatic cells and inhibiting the immune response. Our study demonstrated that increased α-FP is a powerful predictor of prognosis in HCC patients. Cirrhosis was not a predisposing factor for HCC recurrence or patient death in multivariate analysis. Both major LR and α-FP of >40 ng/dL were associated strongly with HCC recurrence and long hospitalization and α-FP level of >40 ng/mL were predisposing factors for patient death. In the non-cirrhotic group, high APRI was associated with HCC recurrence. In addition, antiviral use and α-FP of >40 ng/mL were prognostic factors for death. Antiviral therapies cannot fully eradicate liver cancer risk, especially in HCV-cured patients with advanced liver disease [26]. These findings suggest that there is an accumulation of irreversible damage in the liver during long-term HCV infection. Antiviral therapy is considered a risk factor for death in non-cirrhotic patients. In the cirrhotic group, intraoperative RBC transfusion was associated with HCC recurrence and death. In addition, a long operation time or prolonged hospitalization were predisposing factors for death. Although the non-cirrhotic group had larger tumors and higherα-FP levels than the cirrhotic group, the non-cirrhotic group had better DFS. The OS of the non-cirrhotic group and the cirrhotic group showed little difference until 5 years after surgery. However, the OS of the cirrhotic group decreased rapidly at 5 years after LR.
Liver function is a major determinant of postoperative outcome. Bleeding represents a major source of morbidity during LR and often is managed with RBC transfusions. There is large variation in the proportion of patients who receive RBC transfusions during LR; previous studies reported that 25.2%–56.8% of patients received an RBC transfusion [2728]. In contrast, the intraoperative RBC transfusion rate in our study was 3.4% in the non-cirrhotic group and 5.5% in the cirrhotic group. Suboptimal LR, inadequate division plane, and injury to the major vessels can cause blood loss during LR. These complications can lead to severe sequelae, such as hepatic failure because of decreased tissue perfusion and oxygenation [101214]. In addition, RBC transfusion can have immunomodulatory and proinflammatory effects [2829]. The HCC guidelines in Korea state that blood transfusion compromises anticancer immunologic mechanisms and increases postoperative recurrence [7]. Therefore, surgeons should reduce blood loss during LR and avoid perioperative RBC transfusion. Our study also demonstrated intraoperative RBC transfusion was a predisposing factor for HCC recurrence and death in the cirrhotic group, but not the non-cirrhotic group. Long operation time is related to intraoperative RBC transfusion [28]. Our study also revealed that patients who received intraoperative RBC transfusions had a very long operation time.
However, in this study, APRI was the only predictive factor of HCC recurrence in non-cirrhotic patients. FIB-4 and ALBI grades were not related to HCC recurrence or patient death. Interestingly, we found that HCC recurrence and patient death differed according to FIB-4 grade, ALBI grade, and APRI were beyond 5 years after LR. These results suggest that HCV patients undergoing LR have compensated liver function. Therefore, these noninvasive fibrosis scoring systems, included FIB-4 grade, ALBI grade, and APRI, might not be appropriate for use in this setting.
This study has several limitations. First, all of the included HCC patients were of Korean descent. The majority of Korean HCC patients are infected with HBV, and the prevalence of HCV infection is lower than that of HBV infection in Korea. Second, this was a retrospective study conducted at a single center. Therefore, our study might have been subject to selection bias regarding patient inclusion. Third, because the screening system for HCC in Korea is well established, many Korean patients are diagnosed with HCC at an earlier stage than in other countries. The utility of noninvasive biomarkers of FIB-4 grade, ALBI grade, and APRI might be limited to patients who are diagnosed early. Fourth, many of our patients received antiviral therapy at other hospitals with unknown antiviral drugs and with unclear sustained virologic response. Therefore, these results must be confirmed in additional trials with heterogeneous populations for generalization.
In conclusion, HCV-infected patients who developed treatment-naïve solitary HCC in the background of a non-cirrhotic liver had significantly better DFS and OS than patients with treatment-naïve solitary HCC with a cirrhotic liver after curative LR. In multivariate analysis, the absence of cirrhosis was not a significant predictor of HCC recurrence or death. The factors that influenced HCC recurrence and death differed between the cirrhotic and non-cirrhotic groups. One important clinical situation that affected death in patients with cirrhosis was a long hospital stay to allow liver function recovery due to prolonged operative time and intraoperative RBC transfusion.
References
1. Park SH, Plank LD, Suk KT, Park YE, Lee J, Choi JH, et al. Trends in the prevalence of chronic liver disease in the Korean adult population, 1998-2017. Clin Mol Hepatol. 2020; 26:209–215. PMID: 31679316.

2. Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014; 61(1 Suppl):S58–S68. PMID: 25443346.

3. Goodgame B, Shaheen NJ, Galanko J, El-Serag HB. The risk of end stage liver disease and hepatocellular carcinoma among persons infected with hepatitis C virus: publication bias? Am J Gastroenterol. 2003; 98:2535–2542. PMID: 14638360.

4. Alotaibi AS, Alghamdi W, Marotta P, Qumosani K. A266 Hepatocellular carcinoma prevalence in non-cirrhotic hepat it is C pat ients. J Can Assoc Gastroenterol. 2018; 1(Suppl 2):383–384.
5. Trevisani F, Frigerio M, Santi V, Grignaschi A, Bernardi M. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis. 2010; 42:341–347. PMID: 19828388.

6. Kim JM, Kwon CH, Joh JW, Park JB, Lee JH, Kim SJ, et al. Differences between hepatocellular carcinoma and hepatitis B virus infection in patients with and without cirrhosis. Ann Surg Oncol. 2014; 21:458–465. PMID: 24132624.

7. Korean Liver Cancer Association. National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver. 2019; 13:227–299. PMID: 31060120.
8. Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999; 229:210–215. PMID: 10024102.
9. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003; 38:518–526. PMID: 12883497.

10. Zhang Y, Wang R, Yang X. FIB-4 index serves as a noninvasive prognostic biomarker in patients with hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore). 2018; 97:e13696. PMID: 30572498.
11. Jin W, Lin Z, Xin Y, Jiang X, Dong Q, Xuan S. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis B-related fibrosis: a leading meta-analysis. BMC Gastroenterol. 2012; 12:14. PMID: 22333407.

12. Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD, Xiang BD, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016; 103:725–734. PMID: 27005482.

13. Zou H, Wen Y, Yuan K, Miao XY, Xiong L, Liu KJ. Combining albumin-bilirubin score with future liver remnant predicts post-hepatectomy liver failure in HBV-associated HCC patients. Liver Int. 2018; 38:494–502. PMID: 28685924.

14. Cheng J, Zhao P, Liu J, Liu X, Wu X. Preoperative aspartate aminotransferase-to-platelet ratio index (APRI) is a predictor on postoperative outcomes of hepatocellular carcinoma. Medicine (Baltimore). 2016; 95:e5486. PMID: 27902606.

15. Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A. A laboratory marker, FIB-4 index, as a predictor for long-term outcomes of hepatocellular carcinoma patients after curative hepatic resection. Surgery. 2015; 157:699–707. PMID: 25704421.
16. Hung HH, Su CW, Lai CR, Chau GY, Chan CC, Huang YH, et al. Fibrosis and AST to platelet ratio index predict post-operative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol Int. 2010; 4:691–699. PMID: 21286339.

17. Kim WR, Berg T, Asselah T, Flisiak R, Fung S, Gordon SC, et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016; 64:773–780. PMID: 26626497.

18. Kim JM, Kwon CH, Joh JW, Park JB, Lee JH, Kim SJ, et al. Outcomes after curative hepatectomy in patients with non-B non-C hepatocellular carcinoma and hepatitis B virus hepatocellular carcinoma from non-cirrhotic liver. J Surg Oncol. 2014; 110:976–981. PMID: 25171344.

19. Sinn DH, Lee HW, Paik YH, Kim DY, Kim YJ, Kim KM, et al. Patterns and outcomes in hepatocellular carcinoma patients with portal vein invasion: a multicenter prospective cohort study. Dig Dis Sci. 2021; 66:315–324. PMID: 32056090.

20. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J Hepatol. 2018; 69:154–181. PMID: 29628280.
21. Oh IS, Sinn DH, Kang TW, Lee MW, Kang W, Gwak GY, et al. Liver function assessment using albumin-bilirubin grade for patients with very early-stage hepatocellular carcinoma treated with radiofrequency ablation. Dig Dis Sci. 2017; 62:3235–3242. PMID: 28983724.

22. Kim JM, Kwon CH, Yoo H, Kim KS, Lee J, Kim K, et al. Which approach is preferred in left hepatocellular carcinoma?: laparoscopic versus open hepatectomy using propensity score matching. BMC Cancer. 2018; 18:668. PMID: 29921239.

23. Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in non-cirrhotic liver: a comprehensive review. World J Hepatol. 2019; 11:1–18. PMID: 30705715.

24. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43:1317–1325. PMID: 16729309.

25. Ahn KS, Kang KJ. Appropriate treatment modality for solitary small hepatocellular carcinoma: radiofrequency ablation vs. resection vs. transplantation? Clin Mol Hepatol. 2019; 25:354–359. PMID: 31006225.

26. Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019; 393:1453–1464. PMID: 30765123.

27. Bennett S, Baker LK, Martel G, Shorr R, Pawlik TM, Tinmouth A, et al. The impact of perioperative red blood cell transfusions in patients undergoing liver resection: a systematic review. HPB (Oxford). 2017; 19:321–330. PMID: 28161216.

28. Hallet J, Mahar AL, Nathens AB, Tsang ME, Beyfuss KA, Lin Y, et al. The impact of perioperative blood transfusions on short-term outcomes following hepatectomy. Hepatobiliary Surg Nutr. 2018; 7:1–10. PMID: 29531938.

29. Xun Y, Tian H, Hu L, Yan P, Yang K, Guo T. The impact of perioperative allogeneic blood transfusion on prognosis of hepatocellular carcinoma after radical hepatectomy: a systematic review and meta-analysis of cohort studies. Medicine (Baltimore). 2018; 97:e12911. PMID: 30412094.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1–3 can be found via https://doi.org/10.4174/astr.2022.102.1.1.
Supplementary Table 1
Risk factors for HCC recurrence and mortality in all patients by univariate analysis
Supplementary Table 2
Risk factors for HCC recurrence and mortality in non-cirrhotic patients by univariate analysis
Supplementary Table 3
Risk factors for HCC recurrence and mortality in cirrhotic patients by univariate analysis
Fig. 1
Disease-free survival (DFS) in non-cirrhotic and cirrhotic groups of HCV-related hepatocellular carcinoma (HCC).
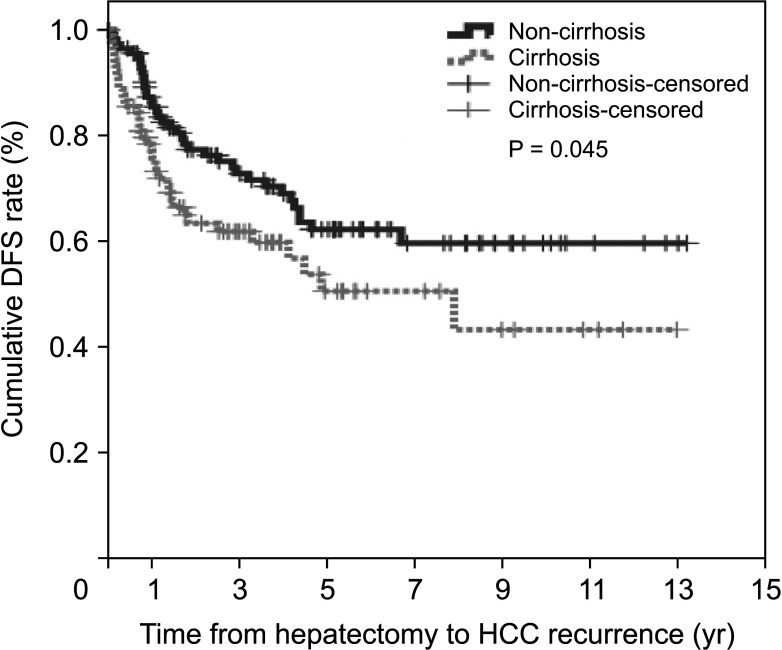
Fig. 2
Overall survival (OS) in non-cirrhotic and cirrhotic groups of HCV-related hepatocellular carcinoma.
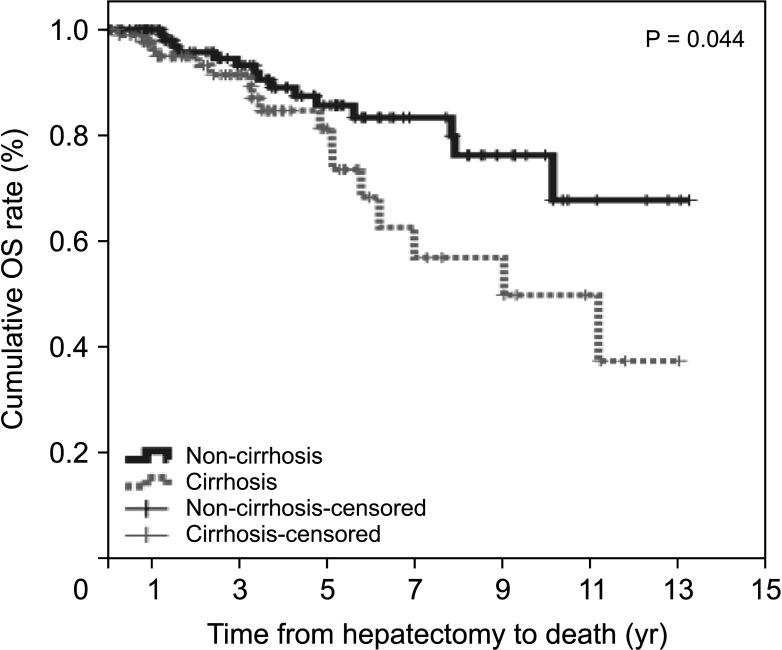
Fig. 3
Hepatocellular carcinoma (HCC) recurrence according to noninvasive markers. (A) Fibrosis-4 (FIB-4) index, (B) AST-to-platelet ratio index (APRI), and (C) albumin-bilirubin (ALBI) grade. DFS, disease-free survival.
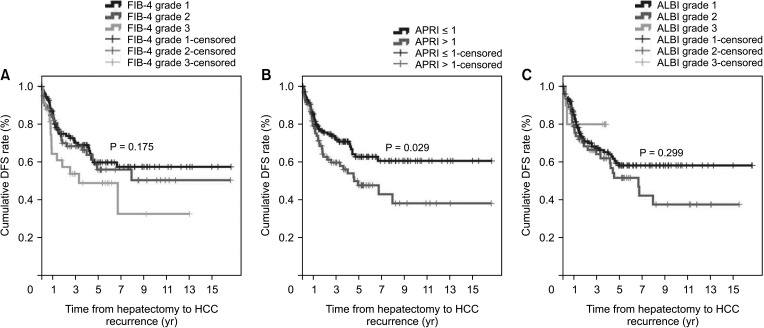
Fig. 4
Overall survival (OS) according to noninvasive markers. (A) Fibrosis-4 (FIB-4) index, (B) AST-to-platelet ratio index (APRI), and (C) albumin-bilirubin (ALBI) grade.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download